Helminth cysteine proteases inhibit TRIF-dependent activation of macrophages via degradation of TLR3
- PMID: 19923225
- PMCID: PMC2823461
- DOI: 10.1074/jbc.M109.060368
Helminth cysteine proteases inhibit TRIF-dependent activation of macrophages via degradation of TLR3
Abstract
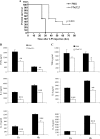
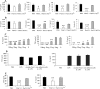
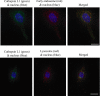
Helminth pathogens prepare a Th2 type immunological environment in their hosts to ensure their longevity. They achieve this by secreting molecules that not only actively drive type 2 responses but also suppress type 1 responses. Here, we show that the major cysteine proteases secreted from the helminth pathogens Fasciola hepatica (FheCL1) and Schistosoma mansoni (SmCB1) protect mice from the lethal effects of lipopolysaccharide by preventing the release of inflammatory mediators, nitric oxide, interleukin-6, tumor necrosis factor alpha, and interleukin-12, from macrophages. The proteases specifically block the MyD88-independent TRIF-dependent signaling pathway of Toll-like receptor (TLR)4 and TLR3. Microscopical and flow cytometric studies, however, show that alteration of macrophage function by cysteine protease is not mediated by cleavage of components of the TLR4 complex on the cell surface but occurs by degradation of TLR3 within the endosome. This is the first study to describe a parasite molecule that degrades this receptor and pinpoints a novel mechanism by which helminth parasites modulate the innate immune responses of their hosts to suppress the development of Th1 responses.
Figures




References
-
- Balic A., Harcus Y. M., Taylor M. D., Brombacher F., Maizels R. M. (2006) Int. Immunol. 18, 1421–1431 - PubMed
-
- Kuroda E., Yoshida Y., En Shan B., Yamashita U. (2001) Parasite Immunol. 23, 305–311 - PubMed
-
- Goodridge H. S., Marshall F. A., Else K. J., Houston K. M., Egan C., Al-Riyami L., Liew F. Y., Harnett W., Harnett M. M. (2005) J. Immunol. 174, 284–293 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

