Vesicle pool size at the salamander cone ribbon synapse
- PMID: 19923246
- PMCID: PMC2807230
- DOI: 10.1152/jn.00718.2009
Vesicle pool size at the salamander cone ribbon synapse
Abstract
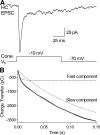
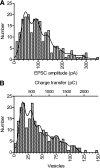
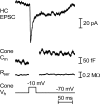
Cone light responses are transmitted to postsynaptic neurons by changes in the rate of synaptic vesicle release. Vesicle pool size at the cone synapse constrains the amount of release and can thus shape contrast detection. We measured the number of vesicles in the rapidly releasable and reserve pools at cone ribbon synapses by performing simultaneous whole cell recording from cones and horizontal or off bipolar cells in the salamander retinal slice preparation. We found that properties of spontaneously occurring miniature excitatory postsynaptic currents (mEPSCs) are representative of mEPSCs evoked by depolarizing presynaptic stimulation. Strong, brief depolarization of the cone stimulated release of the entire rapidly releasable pool (RRP) of vesicles. Comparing charge transfer of the EPSC with mEPSC charge transfer, we determined that the fast component of the EPSC reflects release of approximately 40 vesicles. Comparing EPSCs with simultaneous presynaptic capacitance measurements, we found that horizontal cell EPSCs constitute 14% of the total number of vesicles released from a cone terminal. Using a fluorescent ribeye-binding peptide, we counted approximately 13 ribbons per cone. Together, these results suggest each cone contacts a single horizontal cell at approximately 2 ribbons. The size of discrete components in the EPSC amplitude histogram also suggested approximately 2 ribbon contacts per cell pair. We therefore conclude there are approximately 20 vesicles per ribbon in the RRP, similar to the number of vesicles contacting the plasma membrane at the ribbon base. EPSCs evoked by lengthy depolarization suggest a reserve pool of approximately 90 vesicles per ribbon, similar to the number of additional docking sites further up the ribbon.
Figures
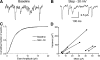



References
-
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in primate retina: a Golgi-electron microscope study of spectral connectivity. J Comp Neurol 343: 406–427, 1994 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

