APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle
- PMID: 19923287
- PMCID: PMC2849269
- DOI: 10.1523/JNEUROSCI.1546-09.2009
APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle
Abstract
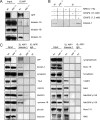
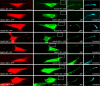
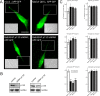
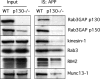
The amyloid precursor protein (APP) is anterogradely transported by conventional kinesin in a distinct transport vesicle, but both the biochemical composition of such a vesicle and the specific kinesin-1 motor responsible for transport are poorly defined. APP may be sequentially cleaved by beta- and gamma-secretases leading to accumulation of beta-amyloid (Abeta) peptides in brains of Alzheimer's disease patients, whereas cleavage of APP by alpha-secretases prevents Abeta generation. Here, we demonstrate by time-lapse analysis and immunoisolations that APP is a cargo of a vesicle containing the kinesin heavy chain isoform kinesin-1C, the small GTPase Rab3A, and a specific subset of presynaptic protein components. Moreover, we report that assembly of kinesin-1C and APP in this vesicle type requires Rab3A GTPase activity. Finally, we show cleavage of APP in transport vesicles by alpha-secretase activity, likely mediated by ADAM10. Together, these data indicate that maturation of APP transport vesicles, including recruitment of conventional kinesin, requires Rab3 GTPase activity.
Figures



Similar articles
-
Presenilin controls kinesin-1 and dynein function during APP-vesicle transport in vivo.Hum Mol Genet. 2013 Oct 1;22(19):3828-43. doi: 10.1093/hmg/ddt237. Epub 2013 May 24. Hum Mol Genet. 2013. PMID: 23710041 Free PMC article.
-
The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport.EMBO J. 2007 Mar 21;26(6):1475-86. doi: 10.1038/sj.emboj.7601609. Epub 2007 Mar 1. EMBO J. 2007. PMID: 17332754 Free PMC article.
-
Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited.J Neurosci. 2005 Mar 2;25(9):2386-95. doi: 10.1523/JNEUROSCI.3089-04.2005. J Neurosci. 2005. PMID: 15745965 Free PMC article.
-
Activation of α-secretase cleavage.J Neurochem. 2012 Jan;120 Suppl 1:46-54. doi: 10.1111/j.1471-4159.2011.07459.x. Epub 2011 Nov 28. J Neurochem. 2012. PMID: 21883223 Review.
-
Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer's disease.Neurodegener Dis. 2006;3(4-5):218-26. doi: 10.1159/000095259. Neurodegener Dis. 2006. PMID: 17047360 Review.
Cited by
-
Preliminary evaluation of the proteomic profiling in the hippocampus of aged grazing cattle.Front Aging Neurosci. 2023 Oct 27;15:1274073. doi: 10.3389/fnagi.2023.1274073. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37965495 Free PMC article.
-
Kinesin-1 Proteins KIF5A, -5B, and -5C Promote Anterograde Transport of Herpes Simplex Virus Enveloped Virions in Axons.J Virol. 2018 Sep 26;92(20):e01269-18. doi: 10.1128/JVI.01269-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30068641 Free PMC article.
-
Trafficking and proteolytic processing of APP.Cold Spring Harb Perspect Med. 2012 May;2(5):a006270. doi: 10.1101/cshperspect.a006270. Cold Spring Harb Perspect Med. 2012. PMID: 22553493 Free PMC article. Review.
-
Phosphorylation-regulated axonal dependent transport of syntaxin 1 is mediated by a Kinesin-1 adapter.Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5862-7. doi: 10.1073/pnas.1113819109. Epub 2012 Mar 26. Proc Natl Acad Sci U S A. 2012. PMID: 22451907 Free PMC article.
-
Increased expression of reticulon 3 in neurons leads to reduced axonal transport of β site amyloid precursor protein-cleaving enzyme 1.J Biol Chem. 2013 Oct 18;288(42):30236-30245. doi: 10.1074/jbc.M113.480079. Epub 2013 Sep 4. J Biol Chem. 2013. PMID: 24005676 Free PMC article.
References
-
- Ali BR, Seabra MC. Targeting of Rab GTPases to cellular membranes. Biochem Soc Trans. 2005;33:652–656. - PubMed
-
- Amaratunga A, Morin PJ, Kosik KS, Fine RE. Inhibition of kinesin synthesis and rapid anterograde axonal transport in vivo by an antisense oligonucleotide. J Biol Chem. 1993;268:17427–17430. - PubMed
-
- Back S, Haas P, Tschäpe JA, Gruebl T, Kirsch J, Müller U, Beyreuther K, Kins S. beta-amyloid precursor protein can be transported independent of any sorting signal to the axonal and dendritic compartment. J Neurosci Res. 2007;85:2580–2590. - PubMed
-
- Burstein ES, Brondyk WH, Macara IG, Kaibuchi K, Takai Y. Regulation of the GTPase cycle of the neuronally expressed Ras-like GTP-binding protein Rab3A. J Biol Chem. 1993;268:22247–22250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
