Magnetic resonance imaging of hypoxic injury to the murine placenta
- PMID: 19923363
- PMCID: PMC2828172
- DOI: 10.1152/ajpregu.00425.2009
Magnetic resonance imaging of hypoxic injury to the murine placenta
Abstract
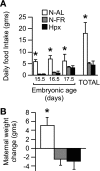
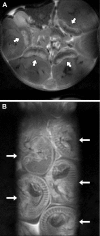
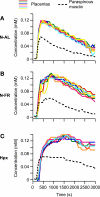
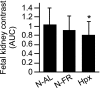
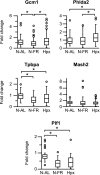
We assessed the use of magnetic resonance imaging (MRI) to define placental hypoxic injury associated with fetal growth restriction. On embryonic day 18.5 (E18.5) we utilized dynamic contrast-enhanced (DCE)-MRI on a 4.7-tesla small animal scanner to examine the uptake and distribution of gadolinium-based contrast agent. Quantitative DCE parameter analysis was performed for the placenta and fetal kidneys of three groups of pregnant C57BL/6 mice: 1) mice that were exposed to Fi(O(2)) = 12% between E15.5 and E18.5, 2) mice in normoxia with food restriction similar to the intake of hypoxic mice between E15.5 and E18.5, and 3) mice in normoxia that were fed ad libitum. After imaging, we assessed fetoplacental weight, placental histology, and gene expression. We found that dams exposed to hypoxia exhibited fetal growth restriction (weight reduction by 28% and 14%, respectively, P < 0.05) with an increased placental-to-fetal ratio. By using MRI-based assessment of placental contrast agent kinetics, referenced to maternal paraspinous muscle, we found decreased placental clearance of contrast media in hypoxic mice, compared with either control group (61%, P < 0.05). This was accompanied by diminished contrast accumulation in the hypoxic fetal kidneys (23%, P < 0.05), reflecting reduced transplacental gadolinium transport. These changes were associated with increased expression of placental Phlda2 and Gcm1 transcripts. Exposure to hypoxia near the end of mouse pregnancy reduces placental perfusion and clearance of contrast. MRI-based DCE imaging provides a novel tool for dynamic, in vivo assessment of placental function.
Figures


References
-
- Abramowicz JS, Sheiner E. In utero imaging of the placenta: importance for diseases of pregnancy. Placenta 28, Suppl A: S14–S22 , 2007 - PubMed
-
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 250: 358– 373, 2002 - PubMed
-
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 25: 311– 314, 2000 - PubMed
-
- Apostolidou S, Abu-Amero S, O'Donoghue K, Frost J, Olafsdottir O, Chavele KM, Whittaker JC, Loughna P, Stanier P, Moore GE. Elevated placental expression of the imprinted PHLDA2 gene is associated with low birth weight. J Mol Med 85: 379– 387, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

