Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis
- PMID: 19923445
- PMCID: PMC2881945
- DOI: 10.4049/jimmunol.0902318
Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis
Erratum in
- J Immunol. 2010 Feb 1;184(3):1656
Abstract
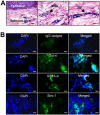
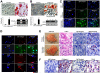
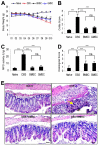
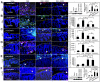
Aside from the well-established self-renewal and multipotent differentiation properties, mesenchymal stem cells exhibit both immunomodulatory and anti-inflammatory roles in several experimental autoimmune and inflammatory diseases. In this study, we isolated a new population of stem cells from human gingiva, a tissue source easily accessible from the oral cavity, namely, gingiva-derived mesenchymal stem cells (GMSCs), which exhibited clonogenicity, self-renewal, and multipotent differentiation capacities. Most importantly, GMSCs were capable of immunomodulatory functions, specifically suppressed peripheral blood lymphocyte proliferation, induced expression of a wide panel of immunosuppressive factors including IL-10, IDO, inducible NO synthase (iNOS), and cyclooxygenase 2 (COX-2) in response to the inflammatory cytokine, IFN-gamma. Cell-based therapy using systemic infusion of GMSCs in experimental colitis significantly ameliorated both clinical and histopathological severity of the colonic inflammation, restored the injured gastrointestinal mucosal tissues, reversed diarrhea and weight loss, and suppressed the overall disease activity in mice. The therapeutic effect of GMSCs was mediated, in part, by the suppression of inflammatory infiltrates and inflammatory cytokines/mediators and the increased infiltration of regulatory T cells and the expression of anti-inflammatory cytokine IL-10 at the colonic sites. Taken together, GMSCs can function as an immunomodulatory and anti-inflammatory component of the immune system in vivo and is a promising cell source for cell-based treatment in experimental inflammatory diseases.
Figures



References
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. - PubMed
-
- Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004;6:1082–1093. - PubMed
-
- Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

