Diagnosis of congenital toxoplasmosis by using a whole-blood gamma interferon release assay
- PMID: 19923492
- PMCID: PMC2812304
- DOI: 10.1128/JCM.01903-09
Diagnosis of congenital toxoplasmosis by using a whole-blood gamma interferon release assay
Abstract
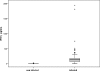
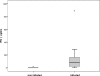
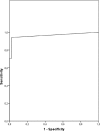
Congenital toxoplasmosis in newborns is generally subclinical, but infected infants are at risk of developing ocular lesions. Diagnosis at birth relies mainly on serological tests. Cell-mediated immunity plays the major role in resistance to infection but is not routinely investigated for diagnostic purposes. Here, we describe a simple test based on the gamma interferon (IFN-gamma) response after stimulation of whole blood by crude parasitic antigens. One milliliter of heparinized blood was centrifuged; plasma was kept for routine serological tests, and pellets were resuspended in culture medium. After 24 h of culture in the presence of crude Toxoplasma gondii antigen, the cells were centrifuged and the supernatant was assayed for IFN-gamma. For 62 infants under 1 year of age born to mothers who were infected during pregnancy, the sensitivity and specificity of the test were 94% (with positive results for 16 of 17 infected infants) and 98% (with negative results for 44 of 45 uninfected infants), respectively. The false-negative result was for a treated baby who gave positive results after the withdrawal of treatment. The false positive was obtained for a 3-month-old baby. For a cohort of 124 congenitally infected patients between 1 and 30 years of age, the sensitivity of the assay was 100%. We present a simple test based on IFN-gamma secretion to assess cell-mediated immunity in toxoplasmosis. As only 1 ml of blood is required to investigate humoral and cellular immunity, our assay is well adapted for the study of congenital toxoplasmosis in infants. Using purified antigens or recombinant peptides may improve the test performance.
Figures

References
-
- Carrara, S., D. Vincenti, N. Petrosillo, M. Amicosante, E. Girardi, and D. Goletti. 2004. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin. Infect. Dis. 38:754-756. - PubMed
-
- Chen, X., B. Zhou, M. Li, Q. Deng, X. Wu, X. Le, C. Wu, N. Larmonier, W. Zhang, H. Zhang, H. Wang, and E. Katsanis. 2007. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 123:50-59. - PubMed
-
- Ciardelli, L., V. Meroni, M. A. Avanzini, L. Bollani, C. Tinelli, F. Garofoli, A. Gasparoni, and M. Stronati. 2008. Early and accurate diagnosis of congenital toxoplasmosis. Pediatr. Infect. Dis. J. 27:125-129. - PubMed
-
- Connell, T., N. Bar-Zeev, and N. Curtis. 2006. Early detection of perinatal tuberculosis using a whole blood interferon-gamma release assay. Clin. Infect. Dis. 42:e82-e85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

