Inflammatory bowel disease
- PMID: 19923578
- PMCID: PMC3491806
- DOI: 10.1056/NEJMra0804647
Inflammatory bowel disease
Figures
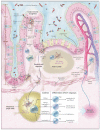
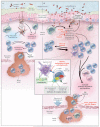
References
-
- Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of non-synonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
