Development of a microfluidics-based intracochlear drug delivery device
- PMID: 19923811
- PMCID: PMC2820330
- DOI: 10.1159/000241898
Development of a microfluidics-based intracochlear drug delivery device
Abstract
Background: Direct delivery of drugs and other agents into the inner ear will be important for many emerging therapies, including the treatment of degenerative disorders and guiding regeneration.
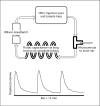
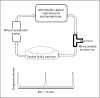
Methods: We have taken a microfluidics/MEMS (MicroElectroMechanical Systems) technology approach to develop a fully implantable reciprocating inner-ear drug-delivery system capable of timed and sequenced delivery of agents directly into perilymph of the cochlea. Iterations of the device were tested in guinea pigs to determine the flow characteristics required for safe and effective delivery. For these tests, we used the glutamate receptor blocker DNQX, which alters auditory nerve responses but not cochlear distortion product otoacoustic emissions.
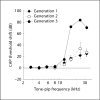
Results: We have demonstrated safe and effective delivery of agents into the scala tympani. Equilibration of the drug in the basal turn occurs rapidly (within tens of minutes) and is dependent on reciprocating flow parameters.
Conclusion: We have described a prototype system for the direct delivery of drugs to the inner ear that has the potential to be a fully implantable means for safe and effective treatment of hearing loss and other diseases.
(c) 2009 S. Karger AG, Basel.
Figures





References
-
- Holley MC. Application of new biological approaches to stimulate sensory repair and protection. Br Med Bull. 2002;63:157–169. - PubMed
-
- Ryan AF, Harris JP, Keithley EM. Immune-mediated hearing loss: basic mechanisms and options for therapy. Acta Otolaryngol Suppl. 2002;548:38–43. - PubMed
-
- Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br Med Bull. 2002;63:25–38. - PubMed
-
- Lalwani AK, Jero J, Mhatre AN. Developments in cochlear gene therapy. Adv Otorhinolaryngol. 2002;61:28–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

