Peroxiredoxin 1 and its role in cell signaling
- PMID: 19923889
- PMCID: PMC7161701
- DOI: 10.4161/cc.8.24.10242
Peroxiredoxin 1 and its role in cell signaling
Abstract
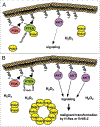
Peroxiredoxins (Prdxs) are a family of small (22-27 kDa) nonseleno peroxidases currently known to possess six mammalian isoforms. Although their individual roles in cellular redox regulation and antioxidant protection are quite distinct, they all catalyze peroxide reduction of H(2)O(2), organic hydroperoxides and peroxynitrite. They are found to be expressed ubiquitously and in high levels, suggesting that they are both an ancient and important enzyme family. Prdxs can be divided into three major subclasses: typical 2-cysteine (2-Cys) Prdxs (Prdx1-4), atypical 2-Cys Prdx (Prdx 5) and 1-Cys Prdx (Prdx 6). Recent evidence suggests that 2-Cys peroxiredoxins are more than "just simple peroxidases". This hypothesis has been discussed elegantly in recent review articles, considering "over"-oxidation of the protonated thiolate peroxidatic cysteine and post-translational modification of Prdxs as processes initiating a mechanistic switch from peroxidase to chaperon function. The process of over-oxidation of the peroxidatic cysteine (C(P)) occurs during catalysis in the presence of thioredoxin (Trx), thus rendering the sulfenic moiety to sulfinic acid, which can be reduced by sulfiredoxin (Srx). However, further oxidation to sulfonic acid is believed to promote Prdx degradation or, as recently shown, the formation of oligomeric peroxidase-inactive chaperones with questionable H(2)O(2)-scavenging capacity. In the light of this and given that Prdx1 has recently been shown by us and by others to interact directly with signaling molecules, we will explore the possibility that H(2)O(2) regulates signaling in the cell in a temporal and spatial fashion via oxidizing Prdx1. Therefore, this review will focus on H(2)O(2) modulating cell signaling via Prdxs by discussing: (1) the activity of Prdxs towards H(2)O(2); (2) sub cellular localization and availability of other peroxidases, such as catalase or glutathione peroxidases; (3) the availability of Prdxs reducing systems, such as thioredoxin and sulfiredoxin and lastly, (4) Prdx1 interacting signaling molecules.
Figures
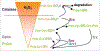
References
-
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 2005; 38:11–7. - PubMed
-
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006; 312:1882–3. - PubMed
-
- Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 2003; 28:32–40. - PubMed
-
- Barranco-Medina S, Lazaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett 2009; 583:1809–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
