Amyloid fibrils of human prion protein are spun and woven from morphologically disordered aggregates
- PMID: 19923901
- PMCID: PMC2807696
- DOI: 10.4161/pri.3.4.10112
Amyloid fibrils of human prion protein are spun and woven from morphologically disordered aggregates
Abstract
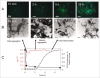
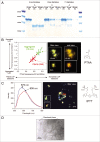
Propagation and infectivity of prions in human prionopathies are likely associated with conversion of the mainly alpha-helical human prion protein, HuPrP, into an aggregated form with amyloid-like properties. Previous reports on efficient conversion of recombinant HuPrP have used mild to harsh denaturing conditions to generate amyloid fibrils in vitro. Herein we report on the in vitro conversion of four forms of truncated HuPrP (sequences 90-231 and 121-231 with and without an N-terminal hexa histidine tag) into amyloid-like fibrils within a few hours by using a protocol (phosphate buffered saline solutions at neutral pH with intense agitation) close to physiological conditions. The conversion process monitored by thioflavin T, ThT, revealed a three stage process with lag, growth and equilibrium phases. Seeding with preformed fibrils shortened the lag phase demonstrating the classic nucleated polymerization mechanism for the reaction. Interestingly, comparing thioflavin T kinetics with solubility and turbidity kinetics it was found that the protein initially formed nonthioflavionophilic, morphologically disordered aggregates that over time matured into amyloid fibrils. By transmission electron microscopy and by fluorescence microscopy of aggregates stained with luminescent conjugated polythiophenes (LCPs); we demonstrated that HuPrP undergoes a conformational conversion where spun and woven fibrils protruded from morphologically disordered aggregates. The initial aggregation functioned as a kinetic trap that decelerated nucleation into a fibrillation competent nucleus, but at the same time without aggregation there was no onset of amyloid fibril formation. The agitation, which was necessary for fibril formation to be induced, transiently exposes the protein to the air-water interface suggests a hitherto largely unexplored denaturing environment for prion conversion.
Figures


References
-
- Westermark P. Aspects on human amyloid forms and their fibril polypeptides. The FEBS journal. 2005;272:5942–5949. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid and human disease. Ann Rev Biochem. 2006;75:333–366. - PubMed
-
- Mishra R, Sorgjerd K, Nystrom S, Nordigarden A, Yu YC, Hammarstrom P. Lysozyme amyloidogenesis is accelerated by specific nicking and fragmentation but decelerated by intact protein binding and conversion. J Mol Biol. 2007;366:1029–1044. - PubMed
-
- Liberski PP. Amyloid plaques in transmissible spongiform encephalopathies (prion diseases) Folia neuropathologica. 2004;42:109–119. - PubMed
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
