Overexpression of CDC14B causes mitotic arrest and inhibits zygotic genome activation in mouse preimplantation embryos
- PMID: 19923902
- PMCID: PMC3188409
- DOI: 10.4161/cc.8.23.10074
Overexpression of CDC14B causes mitotic arrest and inhibits zygotic genome activation in mouse preimplantation embryos
Abstract
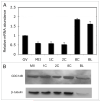
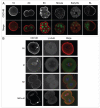
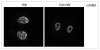
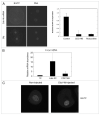
Following fertilization the transition from a highly differentiated oocyte to a totipotent 2-cell embryo requires two unique mitotic cell cycles. The first cell cycle is characterized by a prolonged G(1) phase, DNA replication (S phase) that occurs separately in the female and male pronuclei, and a short G(2) phase that occur in the absence of cell growth. During the second cell cycle, G(1) is short whereas G(2) is prolonged and occurs concurrently with zygotic genome activation, which is essential for progression past the 2-cell stage. CDC14B, a dual specificity phosphatase that counteracts cyclin dependent kinase 1 (CDK1/CDC2A) action, regulates mitosis in somatic cells and prevents premature meiotic resumption in mouse oocytes. It is not known if CDC14B plays a role during the unique mitotic cell cycles of preimplantation development. We report that CDC14B is present in mouse embryos and localizes to mitotic centrosomes and spindles. Overexpressing CDC14B in 1-cell embryos results in 40% and 60% of the embryos arresting at the 1- and 2-cell stages, respectively. Embryos arrested at the 1-cell stage contained reduced CDC2A activity, whereas embryos arrested at the 2-cell stage were in G(2) and failed to activate the zygotic genome. In contrast, overexpressing CDC14B in meiotically-incompetent oocytes, which are arrested in a G(2)-like state and are transcriptionally active, does not repress global transcription. These data suggest that CDC14B is a negative regulator of the 1-to-2-cell transition and of zygotic genome activation in mouse embryogenesis.
Figures



Similar articles
-
CDC14B acts through FZR1 (CDH1) to prevent meiotic maturation of mouse oocytes.Biol Reprod. 2009 Apr;80(4):795-803. doi: 10.1095/biolreprod.108.074906. Epub 2009 Jan 7. Biol Reprod. 2009. PMID: 19129509 Free PMC article.
-
Mis12 controls cyclin B1 stabilization via Cdc14B-mediated APC/CCdh1 regulation during meiotic G2/M transition in mouse oocytes.Development. 2020 Apr 27;147(8):dev185322. doi: 10.1242/dev.185322. Development. 2020. PMID: 32341029
-
The nucleolar phosphatase Cdc14B is dispensable for chromosome segregation and mitotic exit in human cells.Cell Cycle. 2008 May 1;7(9):1184-90. doi: 10.4161/cc.7.9.5792. Epub 2008 Feb 13. Cell Cycle. 2008. PMID: 18418058
-
Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells.Mol Hum Reprod. 2010 Sep;16(9):654-64. doi: 10.1093/molehr/gaq034. Epub 2010 May 7. Mol Hum Reprod. 2010. PMID: 20453035 Free PMC article. Review.
-
[From ovocyte to biochemistry of the cell cycle].Verh K Acad Geneeskd Belg. 1991;53(4):365-85. Verh K Acad Geneeskd Belg. 1991. PMID: 1659057 Review. French.
Cited by
-
Clinical Relevance of Secreted Small Noncoding RNAs in an Embryo Implantation Potential Prediction at Morula and Blastocyst Development Stages.Life (Basel). 2021 Dec 1;11(12):1328. doi: 10.3390/life11121328. Life (Basel). 2021. PMID: 34947859 Free PMC article.
-
The Cdc14B phosphatase contributes to ciliogenesis in zebrafish.Development. 2011 Jan;138(2):291-302. doi: 10.1242/dev.055038. Development. 2011. PMID: 21177342 Free PMC article.
-
Nrf2 inhibition affects cell cycle progression during early mouse embryo development.J Reprod Dev. 2018 Feb 27;64(1):49-55. doi: 10.1262/jrd.2017-042. Epub 2017 Dec 16. J Reprod Dev. 2018. PMID: 29249781 Free PMC article.
-
Characterization of a cdc14 null allele in Drosophila melanogaster.Biol Open. 2018 Jul 9;7(7):bio035394. doi: 10.1242/bio.035394. Biol Open. 2018. PMID: 29945873 Free PMC article.
-
Maternal Setdb1 Is Required for Meiotic Progression and Preimplantation Development in Mouse.PLoS Genet. 2016 Apr 12;12(4):e1005970. doi: 10.1371/journal.pgen.1005970. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27070551 Free PMC article.
References
-
- Sikora-Polaczek M, Hupalowska A, Polanski Z, Kubiak JZ, Ciemerych MA. The first mitosis of the mouse embryo is prolonged by transitional metaphase arrest. Biol Reprod. 2006;74:734–43. - PubMed
-
- Gamow EI, Prescott DM. The cell life cycle during early embryogenesis of the mouse. Exp Cell Res. 1970;59:117–23. - PubMed
-
- Sawicki W, Abramczuk J, Blaton O. DNA synthesis in the second and third cell cycles of mouse preimplantation development. A cytophotometric study. Exp Cell Res. 1978;112:199–205. - PubMed
-
- Luthardt FW, Donahue RP. DNA synthesis in developing two-cell mouse embryos. Dev Biol. 1975;44:210–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
