Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban
- PMID: 19924039
- PMCID: PMC6255425
- DOI: 10.3390/molecules14103922
Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban
Abstract
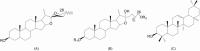
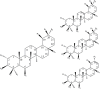
Centella asiatica accumulates large quantities of pentacyclic triterpenoid saponins, collectively known as centelloids. These terpenoids include asiaticoside, centelloside, madecassoside, brahmoside, brahminoside, thankuniside, sceffoleoside, centellose, asiatic-, brahmic-, centellic- and madecassic acids. The triterpene saponins are common secondary plant metabolites and are synthesized via the isoprenoid pathway to produce a hydrophobic triterpenoid structure (aglycone) containing a hydrophilic sugar chain (glycone). The biological activity of saponins has been attributed to these characteristics. In planta, the Centella triterpenoids can be regarded as phytoanticipins due to their antimicrobial activities and protective role against attempted pathogen infections. Preparations of C. asiatica are used in traditional and alternative medicine due to the wide spectrum of pharmacological activities associated with these secondary metabolites. Here, the biosynthesis of the centelloid triterpenoids is reviewed; the range of metabolites found in C. asiatica, together with their known biological activities and the chemotype variation in the production of these metabolites due to growth conditions are summarized. These plant-derived pharmacologically active compounds have complex structures, making chemical synthesis an economically uncompetitive option. Production of secondary metabolites by cultured cells provides a particularly important benefit to manipulate and improve the production of desired compounds; thus biotechnological approaches to increase the concentrations of the metabolites are discussed.
Figures

References
-
- Liu M., van Wyk B.E., Tilney P.-M. A Taxonomic Evaluation of Fruit Structure in the Family Apiaceae. PhD thesis, University of Johannesburg, Auckland Park, South Africa, 2003.
-
- Schaneberg B.T., Mikell J.R., Bedir E., Khan I.A. An improved HPLC method for quantitive determination of six triterpenes in Centella asiatica extracts and commercial products. Pharmazie. 2003;58:381–384. - PubMed
-
- Verma R.K., Bhartariya K.G., Gupta M.M., Kumar S. Reverse-phase high performance liquid chromatography if asiaticoseide in Centella asiatica. Phytochem. Anal. 1999;10:191–193.
-
- Zheng C.-J., Qin L.-P. Chemical components of Centella asiatica and their bioactivities. Chin. J. Integr. Med. 2007;5:348–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

