Rhodanineacetic acid derivatives as potential drugs: preparation, hydrophobic properties and antifungal activity of (5-arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic acids
- PMID: 19924058
- PMCID: PMC6255317
- DOI: 10.3390/molecules14104197
Rhodanineacetic acid derivatives as potential drugs: preparation, hydrophobic properties and antifungal activity of (5-arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic acids
Abstract
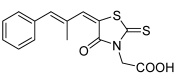
Some [(5Z)-(5-arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)]acetic acids were prepared as potential antifungal compounds. The general synthetic approach to all synthesized compounds is presented. Lipophilicity of all the discussed rhodanine-3-acetic acid derivatives was analyzed using a reversed phase high performance liquid chromatography (RP-HPLC) method. The procedure was performed under isocratic conditions with methanol as an organic modifier in the mobile phase using an end-capped non-polar C(18) stationary RP column. The RP-HPLC retention parameter log k (the logarithm of the capacity factor k) is compared with log P values calculated in silico. All compounds were evaluated for antifungal effects against selected fungal species. Most compounds exhibited no interesting activity, and only {(5Z)-[4-oxo-5-(pyridin-2- ylmethylidene)-2-thioxo-1,3-thiazolidin-3-yl]}acetic acid strongly inhibited the growth of Candida tropicalis 156, Candida krusei E 28, Candida glabrata 20/I and Trichosporon asahii 1188.
Figures


References
-
- Boyd D.B. On the rhodanines and their presence in biologically active ligands. J. Mol. Struct. Theochem. 1997;401:227–234.
-
- Körner H. Derivatives of dithiocarbaminoacetic acid. Ber. Dtsch. Chem. Ges. 1908;41:1901–1905. [Chem. Abstr.1908, 2, 12300]
-
- Andreasch R. Substituted rhodaninic acids and their aldehyde condensation products. VII. Monatsh. Chem. 1908;29:399–419. [Chem. Abstr.1908, 2, 14948]
-
- Taniyama H., Yasui B., Takehara N., Uchida H. Chemotherapeutics for Mycobacterium tuberculosis. XIX. Synthesis and antibacterial activity of some 3-substituted rhodanines. Yakugaku Zasshi. 1959;79:1465–1468. [Chem. Abstr.1960, 54, 34220]
-
- Singh J., Nathan C.F., Bryk R., Samy R., Pupek K., Gurney M. Cyclic carboxylic acid rhodanine derivatives for the treatment and prevention of tuberculosis. WO Pat. 200800. 5651:2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

