Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway
- PMID: 19924065
- PMCID: PMC6255431
- DOI: 10.3390/molecules14114300
Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway
Abstract
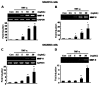
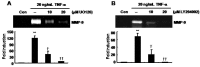
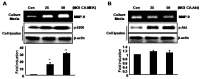
Tumor necrosis factor (TNF)-alpha is one of the pro-inflammatory cytokines highly expressed in Helicobacter pylori that inhibits gastric acid secretion. In this study we determined the effect of silibinin on TNF-alpha-induced MMP-9 expression in gastric cancer cell lines. MMP-9 mRNA and protein expression was dose-dependently increased by TNF-alpha in SNU216 and SNU668 gastric cancer cells. On the other hand, TNF-alpha-induced MMP-9 expression was dose-dependently suppressed by silibinin. To verify the regulatory mechanism of silibinin on TNF-alpha-induced MMP-9 expression, the gastric cancer cell lines were pretreated with silibinin prior to TNF-alpha. TNF-alpha-induced MMP-9 expression was inhibited by the MEK1/2 specific inhibitor, UO126. Finally, we investigated the effect of adenoviral constitutively active (CA)-MEK and CA-Akt on MMP-9 expression. The expression of MMP-9 was significantly increased by CA-MEK overexpression, but not by CA-Akt overexpression. Taken together, we suggest that silibinin down-regulates TNF-alpha- induced MMP-9 expression through inhibition of the MEK/ERK pathway in gastric cancer cells.
Figures

References
-
- Kim S., Choi J.H., Lim H.I., Lee S.K., Kim W.W., Kim J.S., Kim J.H., Choe J.H., Yang J.H., Nam S.J., Lee J.E. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009;16:573–580. doi: 10.1016/j.phymed.2008.11.006. - DOI - PubMed
-
- Sharma G., Singh R.P., Chan D.C., Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23:2649–2655. - PubMed
-
- Agarwal R., Agarwal C., Ichikawa H., Singh R.P., Aggarwal B.B. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

