Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology
- PMID: 19924114
- PMCID: PMC3055598
- DOI: 10.1038/npp.2009.184
Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology
Abstract
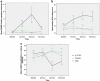
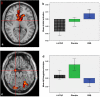
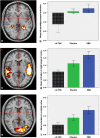
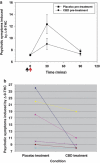
Delta-9-tetrahydrocannabinol (Delta-9-THC) and Cannabidiol (CBD), the two main ingredients of the Cannabis sativa plant have distinct symptomatic and behavioral effects. We used functional magnetic resonance imaging (fMRI) in healthy volunteers to examine whether Delta-9-THC and CBD had opposite effects on regional brain function. We then assessed whether pretreatment with CBD can prevent the acute psychotic symptoms induced by Delta-9-THC. Fifteen healthy men with minimal earlier exposure to cannabis were scanned while performing a verbal memory task, a response inhibition task, a sensory processing task, and when viewing fearful faces. Subjects were scanned on three occasions, each preceded by oral administration of Delta-9-THC, CBD, or placebo. BOLD responses were measured using fMRI. In a second experiment, six healthy volunteers were administered Delta-9-THC intravenously on two occasions, after placebo or CBD pretreatment to examine whether CBD could block the psychotic symptoms induced by Delta-9-THC. Delta-9-THC and CBD had opposite effects on activation relative to placebo in the striatum during verbal recall, in the hippocampus during the response inhibition task, in the amygdala when subjects viewed fearful faces, in the superior temporal cortex when subjects listened to speech, and in the occipital cortex during visual processing. In the second experiment, pretreatment with CBD prevented the acute induction of psychotic symptoms by Delta-9-tetrahydrocannabinol. Delta-9-THC and CBD can have opposite effects on regional brain function, which may underlie their different symptomatic and behavioral effects, and CBD's ability to block the psychotogenic effects of Delta-9-THC.
Figures

References
-
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–451. - PubMed
-
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–973. - PubMed
-
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. - PubMed
-
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

