SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans
- PMID: 19924247
- PMCID: PMC2773845
- DOI: 10.1371/journal.pone.0007792
SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans
Abstract
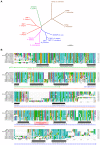
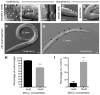
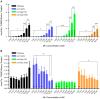
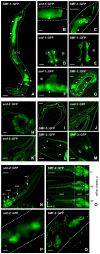
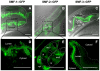
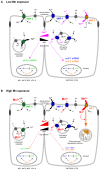
Manganese (Mn) is an essential metal that can exert toxic effects at high concentrations, eventually leading to Parkinsonism. A major transporter of Mn in mammals is the divalent-metal transporter (DMT1). We characterize here DMT1-like proteins in the nematode C. elegans, which regulate and are regulated by Mn and iron (Fe) content. We identified three new DMT1-like genes in C. elegans: smf-1, smf-2 and smf-3. All three can functionally substitute for loss of their yeast orthologues in S. cerevisiae. In the worm, deletion of smf-1 or smf-3 led to an increased Mn tolerance, while loss of smf-2 led to increased Mn sensitivity. smf mRNA levels measured by QRT-PCR were up-regulated upon low Mn and down-regulated upon high Mn exposures. Translational GFP-fusions revealed that SMF-1 and SMF-3 strongly localize to partially overlapping apical regions of the gut epithelium, suggesting a differential role for SMF-1 and SMF-3 in Mn nutritional intake. Conversely, SMF-2 was detected in the marginal pharyngeal epithelium, possibly involved in metal-sensing. Analysis of metal content upon Mn exposure in smf mutants revealed that SMF-3 is required for normal Mn uptake, while smf-1 was dispensable. Higher smf-2 mRNA levels correlated with higher Fe content, supporting a role for SMF-2 in Fe uptake. In smf-1 and smf-3 but not in smf-2 mutants, increased Mn exposure led to decreased Fe levels, suggesting that both metals compete for transport by SMF-2. Finally, SMF-3 was post-translationally and reversibly down-regulated following Mn-exposure. In sum, we unraveled a complex interplay of transcriptional and post-translational regulations of 3 DMT1-like transporters in two adjacent tissues, which regulate metal-content in C. elegans.
Conflict of interest statement
Figures




References
-
- Saric M. Bronchial hyperreactivity and occupational asthma. Am J Ind Med. 1986;9:217–219. - PubMed
-
- Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul. 1984;24:153–169. - PubMed
-
- Addess KJ, Basilion JP, Klausner RD, Rouault TA, Pardi A. Structure and dynamics of the iron responsive element RNA: implications for binding of the RNA by iron regulatory binding proteins. J Mol Biol. 1997;274:72–83. - PubMed
-
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

