Nestedness of ectoparasite-vertebrate host networks
- PMID: 19924299
- PMCID: PMC2774518
- DOI: 10.1371/journal.pone.0007873
Nestedness of ectoparasite-vertebrate host networks
Abstract
Determining the structure of ectoparasite-host networks will enable disease ecologists to better understand and predict the spread of vector-borne diseases. If these networks have consistent properties, then studying the structure of well-understood networks could lead to extrapolation of these properties to others, including those that support emerging pathogens. Borrowing a quantitative measure of network structure from studies of mutualistic relationships between plants and their pollinators, we analyzed 29 ectoparasite-vertebrate host networks--including three derived from molecular bloodmeal analysis of mosquito feeding patterns--using measures of nestedness to identify non-random interactions among species. We found significant nestedness in ectoparasite-vertebrate host lists for habitats ranging from tropical rainforests to polar environments. These networks showed non-random patterns of nesting, and did not differ significantly from published estimates of nestedness from mutualistic networks. Mutualistic and antagonistic networks appear to be organized similarly, with generalized ectoparasites interacting with hosts that attract many ectoparasites and more specialized ectoparasites usually interacting with these same "generalized" hosts. This finding has implications for understanding the network dynamics of vector-born pathogens. We suggest that nestedness (rather than random ectoparasite-host associations) can allow rapid transfer of pathogens throughout a network, and expand upon such concepts as the dilution effect, bridge vectors, and host switching in the context of nested ectoparasite-vertebrate host networks.
Conflict of interest statement
Figures
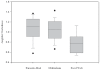
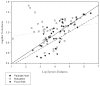

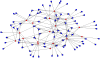

References
-
- Busenberg S, Driessch PVD. Analysis of a disease transmission model in a population with varying size. J Math Biol. 1990;28:1432–1416. - PubMed
-
- Antonovics J, Iwasa Y, Hassell MP. A generalized model of parasitoid, venereal, and vector-based transmission processes. Am Nat. 1995;5:661–675.
-
- Gilot B, Laforge ML, Pinchot J, Raoult D. Relationships between the Rhipicephalus sanguineus complex ecology and Mediterranean spotted fever epidemiology in France. Europ J Epidemiol. 1990;6:357–362. - PubMed
-
- Ostfeld RS, Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can J Zool. 2000;78:2061–2078.
-
- Collinge SK, Ray C. Oxford: Oxford University Press; 2006. Disease Ecology: Community Structure and Pathogen Dynamics.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

