H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment
- PMID: 19924393
- PMCID: PMC7080155
- DOI: 10.1007/s00134-009-1727-6
H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment
Abstract
Purpose: During the 2009 H1N1 influenza A virus pandemic, a minority of patients developed rapidly progressive pneumonia leading to acute lung injury (ALI)-acute respiratory distress syndrome (ARDS). A recent meta-analysis provides support for prolonged corticosteroid treatment in ALI-ARDS. We prospectively evaluated the response to oseltamivir and prolonged corticosteroid treatment in patients with ALI-ARDS and suspected H1N1 influenza.
Methods: From June 24 through 12 July 2009, 13 patients with suspected H1N1 pneumonia and ALI-ARDS were admitted to the intensive care unit (ICU) of a tertiary care hospital. H1N1 influenza was confirmed with real-time reverse transcriptase-polymerase chain reaction assay in eight patients. Oseltamivir and corticosteroid treatment were initiated concomitantly at ICU admission; those with severe ARDS received methylprednisolone (1 mg/kg/day), and others received hydrocortisone (300 mg/day) for a duration of 21 +/- 6 days.
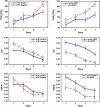
Results: Patients with and without confirmed H1N1 influenza had similar disease severity at presentation and a comparable response to treatment. By day 7 of treatment, patients experienced a significant improvement in lung injury and multiple organ dysfunction scores (P < 0.001). Twelve patients (92%) improved lung function, were extubated, and discharged alive from the ICU. Hospital length of stay and mortality were 18.7 +/- 9.6 days and 15%, respectively. Survivors were discharged home without oxygen supplementation.
Conclusions: In ARDS patients, with and without confirmed H1N1 influenza, prolonged low-to-moderate dose corticosteroid treatment was well tolerated and associated with significant improvement in lung injury and multiple organ dysfunction scores and a low hospital mortality. These findings provide the rationale for developing a randomized trial.
Figures
Comment in
-
Corticosteroids for H1N1 associated acute lung injury: is it just wishful thinking?Intensive Care Med. 2010 Jun;36(6):1098-9; author reply 1100-1. doi: 10.1007/s00134-010-1815-7. Epub 2010 Mar 30. Intensive Care Med. 2010. PMID: 20352192 Free PMC article. No abstract available.
-
Hydrocortisone therapy for patients with H1N1 influenza A infection.Intensive Care Med. 2010 Jun;36(6):1097; author reply 1100-1. doi: 10.1007/s00134-010-1814-8. Epub 2010 Mar 30. Intensive Care Med. 2010. PMID: 20352193 No abstract available.
References
-
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. - DOI - PubMed
-
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA. Intensive-care patients with severe novel influenza A (H1N1) virus infection—Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009;58:749–752. - PubMed
-
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Hospitalized patients with novel influenza A (H1N1) virus infection—California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–541. - PubMed
-
- Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, group THNSw (2009) Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care 13:R148. http://ccforum.com/content/13/5/R148 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

