Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth?
- PMID: 19924633
- PMCID: PMC4497569
- DOI: 10.1387/ijdb.082798ni
Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth?
Abstract
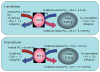
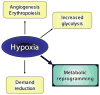
Insufficient oxygen leads to the cessation of growth in favor of cellular survival. Our unique model of high-altitude human pregnancy indicates that hypoxia-induced reductions in fetal growth occur at higher levels of oxygen than previously described. Fetal PO(2) is surprisingly high and fetal oxygen consumption unaffected by high altitude, whereas fetal glucose delivery and consumption decrease. Placental delivery of energy-generating substrates to the fetus is thus altered by mild hypoxia, resulting in maintained fetal oxygenation but a relative fetal hypoglycemia. Our data point to this altered mix of substrates as a potential initiating factor in reduced fetal growth, since oxygen delivery is adequate. These data support the existence, in the placenta, of metabolic reprogramming mechanisms, previously documented in tumor cells, whereby HIF-1 stimulates reductions in mitochondrial oxygen consumption at the cost of increased glucose consumption. Decreased oxygen consumption is not due to substrate (oxygen) limitation but rather results from active inhibition of mitochondrial oxygen utilization. We suggest that under hypoxic conditions, metabolic reprogramming in the placenta decreases mitochondrial oxygen consumption and increases anerobic glucose consumption, altering the mix of energy-generating substrates available for transfer to the fetus. Increased oxygen is available to support the fetus, but at the cost of less glucose availability, leading to a hypoglycemia-mediated decrease in fetal growth. Our data suggest that metabolic reprogramming may be an initiating step in the progression to more severe forms of fetal growth restriction and points to the placenta as the pivotal source of fetal programming in response to an adverse intrauterine environment.
Figures




Similar articles
-
Oxygen delivery and fetal-placental growth: beyond a question of supply and demand?Placenta. 2012 Nov;33 Suppl 2:e16-22. doi: 10.1016/j.placenta.2012.06.006. Epub 2012 Jun 27. Placenta. 2012. PMID: 22742726 Review.
-
Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth.PLoS One. 2010 Jan 1;5(1):e8551. doi: 10.1371/journal.pone.0008551. PLoS One. 2010. PMID: 20049329 Free PMC article.
-
Oxygenation of the placental-fetal unit in humans.Respir Physiol Neurobiol. 2011 Aug 31;178(1):51-8. doi: 10.1016/j.resp.2011.05.009. Epub 2011 May 17. Respir Physiol Neurobiol. 2011. PMID: 21621012 Review.
-
Placental adaptive responses and fetal programming.J Physiol. 2006 Apr 1;572(Pt 1):25-30. doi: 10.1113/jphysiol.2006.104968. Epub 2006 Feb 9. J Physiol. 2006. PMID: 16469781 Free PMC article. Review.
-
Effect of restriction of placental growth on fetal and utero-placental metabolism.J Dev Physiol. 1987 Jun;9(3):225-38. J Dev Physiol. 1987. PMID: 3611639
Cited by
-
Decreased Vitamin D Levels and Altered Placental Vitamin D Gene Expression at High Altitude: Role of Genetic Ancestry.Int J Mol Sci. 2023 Feb 8;24(4):3389. doi: 10.3390/ijms24043389. Int J Mol Sci. 2023. PMID: 36834800 Free PMC article.
-
Gestational differences in murine placenta: Glycolytic metabolism and pregnancy parameters.Theriogenology. 2018 Feb;107:115-126. doi: 10.1016/j.theriogenology.2017.10.049. Epub 2017 Nov 4. Theriogenology. 2018. PMID: 29145065 Free PMC article.
-
Soluble Fms-Like Tyrosine Kinase-1 Alters Cellular Metabolism and Mitochondrial Bioenergetics in Preeclampsia.Front Physiol. 2018 Mar 6;9:83. doi: 10.3389/fphys.2018.00083. eCollection 2018. Front Physiol. 2018. PMID: 29563877 Free PMC article.
-
Adenosine A₂a receptors and O₂ sensing in development.Am J Physiol Regul Integr Comp Physiol. 2011 Sep;301(3):R601-22. doi: 10.1152/ajpregu.00664.2010. Epub 2011 Jun 15. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21677265 Free PMC article. Review.
-
Intrauterine programming.Iran J Basic Med Sci. 2015 Mar;18(3):212-20. Iran J Basic Med Sci. 2015. PMID: 25945232 Free PMC article. Review.
References
-
- AARDEMA MW, OOSTERHOF H, TIMMER A, VAN ROOY I, AARNOUDSE JG. Uterine artery doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by preeclampsia and small for gestational age fetuses. Placenta. 2001;22:405–411. - PubMed
-
- ARAGONES J, SCHNEIDER M, VAN GEYTE K, FRAISL P, DRESSELAERS T, MAZZONE M, DIRKX R, ZACCHIGNA S, LEMIEUX H, JEOUNG NH, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–80. - PubMed
-
- BAGGA R, VASISHTA K, MAJUMDAR S, GARG SK. Correlation between human placental lactogen levels and glucose metabolism in pregnant women with intrauterine growth retardation. Aust N Z J Obstet Gynaecol. 1990;30:310–3. - PubMed
-
- BOWER S, BEWLEY S, CAMPBELL S. Improved prediction of preeclampsia by two-stage screening of uterine arteries using the early diastolic notch and color Doppler imaging. Obstet Gynecol. 1993;82:78–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

