Inhibition of the class C beta-lactamase from Acinetobacter spp.: insights into effective inhibitor design
- PMID: 19925018
- PMCID: PMC2810401
- DOI: 10.1021/bi9015988
Inhibition of the class C beta-lactamase from Acinetobacter spp.: insights into effective inhibitor design
Abstract
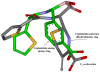
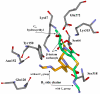
The need to develop beta-lactamase inhibitors against class C cephalosporinases of Gram-negative pathogens represents an urgent clinical priority. To respond to this challenge, five boronic acid derivatives, including a new cefoperazone analogue, were synthesized and tested against the class C cephalosporinase of Acinetobacter baumannii [Acinetobacter-derived cephalosporinase (ADC)]. The commercially available carbapenem antibiotics were also assayed. In the boronic acid series, a chiral cephalothin analogue with a meta-carboxyphenyl moiety corresponding to the C(3)/C(4) carboxylate of beta-lactams showed the lowest K(i) (11 +/- 1 nM). In antimicrobial susceptibility tests, this cephalothin analogue lowered the ceftazidime and cefotaxime minimum inhibitory concentrations (MICs) of Escherichia coli DH10B cells carrying bla(ADC) from 16 to 4 microg/mL and from 8 to 1 microg/mL, respectively. On the other hand, each carbapenem exhibited a K(i) of <20 microM, and timed electrospray ionization mass spectrometry (ESI-MS) demonstrated the formation of adducts corresponding to acyl-enzyme intermediates with both intact carbapenem and carbapenem lacking the C(6) hydroxyethyl group. To improve our understanding of the interactions between the beta-lactamase and the inhibitors, we constructed models of ADC as an acyl-enzyme intermediate with (i) the meta-carboxyphenyl cephalothin analogue and (ii) the carbapenems, imipenem and meropenem. Our first model suggests that this chiral cephalothin analogue adopts a novel conformation in the beta-lactamase active site. Further, the addition of the substituent mimicking the cephalosporin dihydrothiazine ring may significantly improve affinity for the ADC beta-lactamase. In contrast, the ADC-carbapenem models offer a novel role for the R(2) side group and also suggest that elimination of the C(6) hydroxyethyl group by retroaldolic reaction leads to a significant conformational change in the acyl-enzyme intermediate. Lessons from the diverse mechanisms and structures of the boronic acid derivatives and carbapenems provide insights for the development of new beta-lactamase inhibitors against these critical drug resistance targets.
Figures








References
-
- Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. - PubMed
-
- Poirel L, Karim A, Mercat A, Le Thomas I, Vahaboglu H, Richard C, Nordmann P. Extended-spectrum β-lactamase-producing strain of Acinetobacter baumannii isolated from a patient in France. J Antimicrob Chemother. 1999;43:157–158. - PubMed
-
- Cisneros JM, Rodriguez-Bano J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect. 2002;8:687–693. - PubMed
-
- Gootz TD, Marra A. Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev Anti Infect Ther. 2008;6:309–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

