Abnormalities in brain structure and behavior in GSK-3alpha mutant mice
- PMID: 19925672
- PMCID: PMC2785804
- DOI: 10.1186/1756-6606-2-35
Abnormalities in brain structure and behavior in GSK-3alpha mutant mice
Abstract
Background: Glycogen synthase kinase-3 (GSK-3) is a widely expressed and highly conserved serine/threonine protein kinase encoded by two genes that generate two related proteins: GSK-3alpha and GSK-3beta. Mice lacking a functional GSK-3alpha gene were engineered in our laboratory; they are viable and display insulin sensitivity. In this study, we have characterized brain functions of GSK-3alpha KO mice by using a well-established battery of behavioral tests together with neurochemical and neuroanatomical analysis.
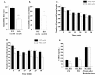
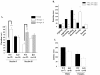
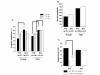
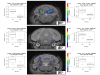
Results: Similar to the previously described behaviours of GSK-3beta(+/-) mice, GSK-3alpha mutants display decreased exploratory activity, decreased immobility time and reduced aggressive behavior. However, genetic inactivation of the GSK-3alpha gene was associated with: decreased locomotion and impaired motor coordination, increased grooming activity, loss of social motivation and novelty; enhanced sensorimotor gating and impaired associated memory and coordination. GSK-3alpha KO mice exhibited a deficit in fear conditioning, however memory formation as assessed by a passive avoidance test was normal, suggesting that the animals are sensitized for active avoidance of a highly aversive stimulus in the fear-conditioning paradigm. Changes in cerebellar structure and function were observed in mutant mice along with a significant decrease of the number and size of Purkinje cells.
Conclusion: Taken together, these data support a role for the GSK-3alpha gene in CNS functioning and possible involvement in the development of psychiatric disorders.
Figures



References
-
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107(2):519–527. - PubMed
-
- Woodgett JR, Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5) Biochim Biophys Acta. 1984;788(3):339–347. - PubMed
-
- Ferrer I, Barrachina M, Puig B. Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2002;104(6):583–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

