Regulation of vascular smooth muscle cell bioenergetic function by protein glutathiolation
- PMID: 19925774
- PMCID: PMC2812626
- DOI: 10.1016/j.bbabio.2009.11.005
Regulation of vascular smooth muscle cell bioenergetic function by protein glutathiolation
Abstract
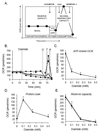
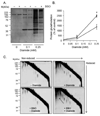
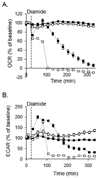
Protein thiolation by glutathione is a reversible and regulated post-translational modification that is increased in response to oxidants and nitric oxide. Because many mitochondrial enzymes contain critical thiol residues, it has been hypothesized that thiolation reactions regulate cell metabolism and survival. However, it has been difficult to differentiate the biological effects due to protein thiolation from other oxidative protein modifications. In this study, we used diamide to titrate protein glutathiolation and examined its impact on glycolysis, mitochondrial function, and cell death in rat aortic smooth muscle cells. Treatment of cells with diamide increased protein glutathiolation in a concentration-dependent manner and had comparably little effect on protein-protein disulfide formation. Diamide increased mitochondrial proton leak and decreased ATP-linked mitochondrial oxygen consumption and cellular bioenergetic reserve capacity. Concentrations of diamide above 200 microM promoted acute bioenergetic failure and caused cell death, whereas lower concentrations of diamide led to a prolonged increase in glycolytic flux and were not associated with loss of cell viability. Depletion of glutathione using buthionine sulfoximine had no effect on basal protein thiolation or cellular bioenergetics but decreased diamide-induced protein glutathiolation and sensitized the cells to bioenergetic dysfunction and death. The effects of diamide on cell metabolism and viability were fully reversible upon addition of dithiothreitol. These data suggest that protein thiolation modulates key metabolic processes in both the mitochondria and cytosol.
2009 Elsevier B.V. All rights reserved.
Figures




References
-
- Diotte NM, Xiong Y, Gao J, Chua BH, Ho YS. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim. Biophys. Acta. 2009;1793:427–438. - PubMed
-
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10:1200–1207. - PubMed
-
- Ghezzi P, Romines B, Fratelli M, Eberini I, Gianazza E, Casagrande S, Laragione T, Mengozzi M, Herzenberg LA, Herzenberg LA. Protein glutathionylation: coupling and uncoupling of glutathione to protein thiol groups in lymphocytes under oxidative stress and HIV infection. Mol. Immunol. 2002;38:773–780. - PubMed
-
- Eaton P, Byers HL, Leeds N, Ward MA, Shattock MJ. Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J. Biol. Chem. 2002;277:9806–9811. - PubMed
-
- Lind C, Gerdes R, Schuppe-Koistinen I, Cotgreave IA. Studies on the mechanism of oxidative modification of human glyceraldehyde-3-phosphate dehydrogenase by glutathione: catalysis by glutaredoxin. Biochem. Biophys. Res. Commun. 1998;247:481–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

