Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla
- PMID: 19926534
- PMCID: PMC2844926
- DOI: 10.1016/j.autneu.2009.10.002
Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla
Abstract
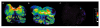
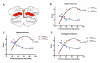
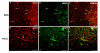
gamma-Amino butyric (GABA) critically influences serotonergic (5-HT) neurons in the raphé and extra-raphé of the medulla oblongata. In this study we hypothesize that there are marked changes in the developmental profile of markers of the human medullary GABAergic system relative to the 5-HT system in early life. We used single- and double-label immunocytochemistry and tissue receptor autoradiography in 15 human medullae from fetal and infant cases ranging from 15 gestational weeks to 10 postnatal months, and compared our findings with an extensive 5-HT-related database in our laboratory. In the raphé obscurus, we identified two subsets of GABAergic neurons using glutamic acid decarboxylase (GAD65/67) immunostaining: one comprised of small, round neurons; the other, medium, spindle-shaped neurons. In three term medullae cases, positive immunofluorescent neurons for both tryptophan hydroxylase and GAD65/67 were counted within the raphé obscurus. This revealed that approximately 6% of the total neurons counted in this nucleus expressed both GAD65/67 and TPOH suggesting co-production of GABA by a subset of 5-HT neurons. The distribution of GABA(A) binding was ubiquitous across medullary nuclei, with highest binding in the raphé obscurus. GABA(A) receptor subtypes alpha1 and alpha3 were expressed by 5-HT neurons, indicating the site of interaction of GABA with 5-HT neurons. These receptor subtypes and KCC2, a major chloride transporter, were differentially expressed across early development, from midgestation (20 weeks) and thereafter. The developmental profile of GABAergic markers changed dramatically relative to the 5-HT markers. These data provide baseline information for medullary studies of human pediatric disorders, such as sudden infant death syndrome.
2009 Elsevier B.V. All rights reserved.
Figures










Similar articles
-
Differential development of 5-HT receptor and the serotonin transporter binding in the human infant medulla.J Comp Neurol. 2004 Apr 26;472(2):221-31. doi: 10.1002/cne.20105. J Comp Neurol. 2004. PMID: 15048689
-
The serotonergic anatomy of the developing human medulla oblongata: implications for pediatric disorders of homeostasis.J Chem Neuroanat. 2011 Jul;41(4):182-99. doi: 10.1016/j.jchemneu.2011.05.004. Epub 2011 May 27. J Chem Neuroanat. 2011. PMID: 21640183 Free PMC article. Review.
-
Multiple serotonergic brainstem abnormalities in sudden infant death syndrome.JAMA. 2006 Nov 1;296(17):2124-32. doi: 10.1001/jama.296.17.2124. JAMA. 2006. PMID: 17077377
-
Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome.J Neuropathol Exp Neurol. 2000 May;59(5):377-84. doi: 10.1093/jnen/59.5.377. J Neuropathol Exp Neurol. 2000. PMID: 10888367
-
Distribution of cholinergic, GABAergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat.Neuroscience. 1994 Oct;62(4):1155-78. doi: 10.1016/0306-4522(94)90351-4. Neuroscience. 1994. PMID: 7845592 Review.
Cited by
-
Dopaminergic neurons inhibit striatal output through non-canonical release of GABA.Nature. 2012 Oct 11;490(7419):262-6. doi: 10.1038/nature11466. Epub 2012 Oct 3. Nature. 2012. PMID: 23034651 Free PMC article.
-
The Serotonin Brainstem Hypothesis for the Sudden Infant Death Syndrome.J Neuropathol Exp Neurol. 2019 Sep 1;78(9):765-779. doi: 10.1093/jnen/nlz062. J Neuropathol Exp Neurol. 2019. PMID: 31397480 Free PMC article. Review.
-
Serotonin metabolites in the cerebrospinal fluid in sudden infant death syndrome.J Neuropathol Exp Neurol. 2014 Feb;73(2):115-22. doi: 10.1097/NEN.0000000000000034. J Neuropathol Exp Neurol. 2014. PMID: 24423636 Free PMC article.
-
Late development of the GABAergic system in the human cerebral cortex and white matter.J Neuropathol Exp Neurol. 2011 Oct;70(10):841-58. doi: 10.1097/NEN.0b013e31822f471c. J Neuropathol Exp Neurol. 2011. PMID: 21937910 Free PMC article.
-
Brainstem deficiency of the 14-3-3 regulator of serotonin synthesis: a proteomics analysis in the sudden infant death syndrome.Mol Cell Proteomics. 2012 Jan;11(1):M111.009530. doi: 10.1074/mcp.M111.009530. Epub 2011 Oct 5. Mol Cell Proteomics. 2012. PMID: 21976671 Free PMC article.
References
-
- Baker KG, Halliday GM, Halasz P, Hornung JP, Geffen LB, Cotton RG, Tork I. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991;7:301–320. - PubMed
-
- Belin MF, Nanopoulos D, Didier M, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF. Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. - PubMed
-
- Bohm I, Xia L, Leiter JC, Bartlett D. GABAergic processes mediate thermal prolongation of the laryngeal reflex apnea in decerebrate piglets. Respir Physiol Neurobiol. 2007;156:229–233. - PubMed
-
- Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Auton Neurosci. 2000;84:58–67. - PubMed
-
- Callera JC, Colombari E, De Luca LA, Jr., Menani JV. The bradycardic and hypotensive responses to serotonin are reduced by activation of GABAA receptors in the nucleus tractus solitarius of awake rats. Braz J Med Biol Res. 2005;38:1123–1131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

