Sporophytic control of pollen tube growth and guidance in maize
- PMID: 19926683
- PMCID: PMC2814102
- DOI: 10.1093/jxb/erp330
Sporophytic control of pollen tube growth and guidance in maize
Abstract
Pollen tube germination, growth, and guidance (progamic phase) culminating in sperm discharge is a multi-stage process including complex interactions between the male gametophyte as well as sporophytic tissues and the female gametophyte (embryo sac), respectively. Inter- and intra-specific crossing barriers in maize and Tripsacum have been studied and a precise description of progamic pollen tube development in maize is reported here. It was found that pollen germination and initial tube growth are rather unspecific, but an early, first crossing barrier was detected before arrival at the transmitting tract. Pollination of maize silks with Tripsacum pollen and incompatible pollination of Ga1s/Ga1s-maize silks with ga1-maize pollen revealed another two incompatibility barriers, namely transmitting tract mistargeting and insufficient growth support. Attraction and growth support by the transmitting tract seem to play key roles for progamic pollen tube growth. After leaving transmitting tracts, pollen tubes have to navigate across the ovule in the ovular cavity. Pollination of an embryo sac-less maize RNAi-line allowed the role of the female gametophyte for pollen tube guidance to be determined in maize. It was found that female gametophyte controlled guidance is restricted to a small region around the micropyle, approximately 50-100 microm in diameter. This area is comparable to the area of influence of previously described ZmEA1-based short-range female gametophyte signalling. In conclusion, the progamic phase is almost completely under sporophytic control in maize.
Figures
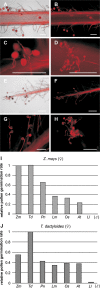




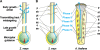
Comment in
-
Establishment of the male germline and sperm cell movement during pollen germination and tube growth in maize.Plant Signal Behav. 2010 Jul;5(7):885-9. doi: 10.4161/psb.5.7.12033. Epub 2010 Jul 1. Plant Signal Behav. 2010. PMID: 20505353 Free PMC article.
References
-
- Arnold ML, Hodges SA. Are natural hybrids fit or unfit relative to their parents. Trends in Ecology and Evolution. 1995;10:67–71. - PubMed
-
- Bedinger PA, Fowler JE. In: The maize male gametophyte. Bennetzen JL, Hake SC, editors. New York: Springer-Verlag, Inc; 2009. pp. 57–77.
-
- Booy G, Krens FA, Bino RJ. Analysis of pollen-tube growth in cultured maize silks. Sexual Plant Reproduction. 1992;5:227–231.
-
- Dresselhaus T, Márton ML. Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Current Opinion in Plant Biology. 2009;12 DOI:10.1016/j.pbi.2009.09.015. - PubMed
-
- Heslop-Harrison J. Pollen–stigma interaction and cross-incompatibility in the grasses. Science. 1982;215:1358–1364. - PubMed

