Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization
- PMID: 19926723
- PMCID: PMC2802038
- DOI: 10.1261/rna.1869710
Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization
Abstract
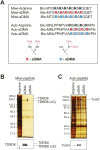
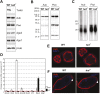
Piwi proteins such as Drosophila Aubergine (Aub) and mouse Miwi are essential for germline development and for primordial germ cell (PGC) specification. They bind piRNAs and contain symmetrically dimethylated arginines (sDMAs), catalyzed by dPRMT5. PGC specification in Drosophila requires maternal inheritance of cytoplasmic factors, including Aub, dPRMT5, and Tudor (Tud), that are concentrated in the germ plasm at the posterior end of the oocyte. Here we show that Miwi binds to Tdrd6 and Aub binds to Tudor, in an sDMA-dependent manner, demonstrating that binding of sDMA-modified Piwi proteins with Tudor-domain proteins is an evolutionarily conserved interaction in germ cells. We report that in Drosophila tud(1) mutants, the piRNA pathway is intact and most transposons are not de-repressed. However, the localization of Aub in the germ plasm is severely reduced. These findings indicate that germ plasm assembly requires sDMA modification of Aub by dPRMT5, which, in turn, is required for binding to Tudor. Our study also suggests that the function of the piRNA pathway in PGC specification may be independent of its role in transposon control.
Figures
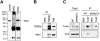

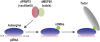
References
-
- Anne J, Mechler BM. Valois, a component of the nuage and pole plasm, is involved in assembly of these structures, and binds to Tudor and the methyltransferase Capsuleen. Development. 2005;132:2167–2177. - PubMed
-
- Anne J, Ollo R, Ephrussi A, Mechler BM. Arginine methyltransferase Capsuleen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development. 2007;134:137–146. - PubMed
-
- Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5:337–350. - PubMed
-
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. - PubMed
-
- Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
