Poly(A) signal-dependent degradation of unprocessed nascent transcripts accompanies poly(A) signal-dependent transcriptional pausing in vitro
- PMID: 19926725
- PMCID: PMC2802029
- DOI: 10.1261/rna.1622010
Poly(A) signal-dependent degradation of unprocessed nascent transcripts accompanies poly(A) signal-dependent transcriptional pausing in vitro
Abstract
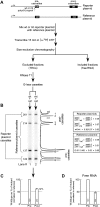
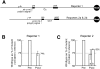
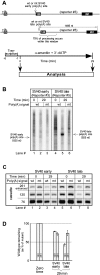
The poly(A) signal has long been known for its role in directing the cleavage and polyadenylation of eukaryotic mRNA. In recent years its additional coordinating role in multiple related aspects of gene expression has also become increasingly clear. Here we use HeLa nuclear extracts to study two of these activities, poly(A) signal-dependent transcriptional pausing, which was originally proposed as a surveillance checkpoint, and poly(A) signal-dependent degradation (PDD) of unprocessed transcripts from weak poly(A) signals. We confirm directly, by measuring the length of RNA within isolated transcription elongation complexes, that a newly transcribed poly(A) signal reduces the rate of elongation by RNA polymerase II and causes the accumulation of elongation complexes downstream from the poly(A) signal. We then show that if the RNA in these elongation complexes contains a functional but unprocessed poly(A) signal, degradation of the transcripts ensues. The degradation depends on the unprocessed poly(A) signal being functional, and does not occur if a mutant poly(A) signal is used. We suggest that during normal 3'-end processing the uncleaved poly(A) signal continuously samples competing reaction pathways for processing and for degradation, and that in the case of weak poly(A) signals, where poly(A) site cleavage is slow, the default pathway to degradation predominates.
Figures



Similar articles
-
Specific transcriptional pausing activates polyadenylation in a coupled in vitro system.Mol Cell. 1999 May;3(5):593-600. doi: 10.1016/s1097-2765(00)80352-4. Mol Cell. 1999. PMID: 10360175
-
Human Pcf11 enhances degradation of RNA polymerase II-associated nascent RNA and transcriptional termination.Nucleic Acids Res. 2008 Feb;36(3):905-14. doi: 10.1093/nar/gkm1112. Epub 2007 Dec 17. Nucleic Acids Res. 2008. PMID: 18086705 Free PMC article.
-
The RNA tether from the poly(A) signal to the polymerase mediates coupling of transcription to cleavage and polyadenylation.Mol Cell. 2005 Dec 9;20(5):733-45. doi: 10.1016/j.molcel.2005.09.026. Mol Cell. 2005. PMID: 16337597
-
On the importance of being co-transcriptional.J Cell Sci. 2002 Oct 15;115(Pt 20):3865-71. doi: 10.1242/jcs.00073. J Cell Sci. 2002. PMID: 12244124 Review.
-
mRNA polyadenylation and its coupling to other RNA processing reactions and to transcription.Curr Opin Cell Biol. 1999 Jun;11(3):352-7. doi: 10.1016/S0955-0674(99)80049-0. Curr Opin Cell Biol. 1999. PMID: 10395555 Review.
Cited by
-
Coupled RNA polymerase II transcription and 3' end formation with yeast whole-cell extracts.RNA. 2010 Nov;16(11):2205-17. doi: 10.1261/rna.2172510. Epub 2010 Sep 1. RNA. 2010. PMID: 20810619 Free PMC article.
-
A complex immunodeficiency is based on U1 snRNP-mediated poly(A) site suppression.EMBO J. 2012 Oct 17;31(20):4035-44. doi: 10.1038/emboj.2012.252. Epub 2012 Sep 11. EMBO J. 2012. PMID: 22968171 Free PMC article.
-
RNA polymerase II pausing downstream of core histone genes is different from genes producing polyadenylated transcripts.PLoS One. 2012;7(6):e38769. doi: 10.1371/journal.pone.0038769. Epub 2012 Jun 11. PLoS One. 2012. PMID: 22701709 Free PMC article.
-
A model in vitro system for co-transcriptional splicing.Nucleic Acids Res. 2010 Nov;38(21):7570-8. doi: 10.1093/nar/gkq620. Epub 2010 Jul 14. Nucleic Acids Res. 2010. PMID: 20631007 Free PMC article.
-
Temporal Dissection of Rate Limiting Transcriptional Events Using Pol II ChIP and RNA Analysis of Adrenergic Stress Gene Activation.PLoS One. 2015 Aug 5;10(8):e0134442. doi: 10.1371/journal.pone.0134442. eCollection 2015. PLoS One. 2015. PMID: 26244980 Free PMC article.
References
-
- Andrulis ED, Werner J, Nazarian A, Erdjument-Bromage H, Tempst P, Lis JT. The RNA processing exosome is linked to elongating RNA polymerase II in Drosophila. Nature. 2002;420:837–841. - PubMed
-
- Calvo O, Manley JL. Strange bedfellows: Polyadenylation factors at the promoter. Genes & Dev. 2003;17:1321–1327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
