The ClC-3 Cl-/H+ antiporter becomes uncoupled at low extracellular pH
- PMID: 19926787
- PMCID: PMC2807314
- DOI: 10.1074/jbc.M109.018002
The ClC-3 Cl-/H+ antiporter becomes uncoupled at low extracellular pH
Abstract
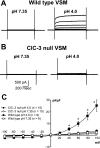
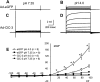
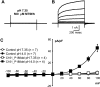
Adenovirus expressing ClC-3 (Ad-ClC-3) induces Cl(-)/H(+) antiport current (I(ClC-3)) in HEK293 cells. The outward rectification and time dependence of I(ClC-3) closely resemble an endogenous HEK293 cell acid-activated Cl(-) current (ICl(acid)) seen at extracellular pH <or= 5.5. ICl(acid) was present in smooth muscle cells from wild-type but not ClC-3 null mice. We therefore sought to determine whether these currents were related. ICl(acid) was larger in cells expressing Ad-ClC-3. Protons shifted the reversal potential (E(rev)) of I(ClC-3) between pH 8.2 and 6.2, but not pH 6.2 and 5.2, suggesting that Cl(-) and H(+) transport become uncoupled at low pH. At pH 4.0 E(rev) was completely Cl(-) dependent (55.8 +/- 2.3 mV/decade). Several findings linked ClC-3 with native ICl(acid); 1) RNA interference directed at ClC-3 message reduced native ICl(acid); 2) removal of the extracellular "fast gate" (E224A) produced large currents that were pH-insensitive; and 3) wild-type I(ClC-3) and ICl(acid) were both inhibited by (2-sulfonatoethyl)methanethiosulfonate (MTSES; 10-500 microm)-induced alkanethiolation at exposed cysteine residues. However, a ClC-3 mutant lacking four extracellular cysteine residues (C103_P130del) was completely resistant to MTSES. C103_P130del currents were still acid-activated, but could be distinguished from wild-type I(ClC-3) and from native ICl(acid) by a much slower response to low pH. Thus, ClC-3 currents are activated by protons and ClC-3 protein may account for native ICl(acid). Low pH uncouples Cl(-)/H(+) transport so that at pH 4.0 ClC-3 behaves as an anion-selective channel. These findings have important implications for the biology of Cl(-)/H(+) antiporters and perhaps for pH regulation in highly acidic intracellular compartments.
Figures





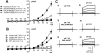
References
-
- Jentsch T. J., Poët M., Fuhrmann J. C., Zdebik A. A. (2005) Annu. Rev. Physiol. 67, 779–807 - PubMed
-
- Friedrich T., Breiderhoff T., Jentsch T. J. (1999) J. Biol. Chem. 274, 896–902 - PubMed
-
- Weylandt K. H., Valverde M. A., Nobles M., Raguz S., Amey J. S., Diaz M., Nastrucci C., Higgins C. F., Sardini A. (2001) J. Biol. Chem. 276, 17461–17467 - PubMed
-
- Duan D., Winter C., Cowley S., Hume J. R., Horowitz B. (1997) Nature 390, 417–421 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

