Hypercholesterolemia induces side-specific phenotypic changes and peroxisome proliferator-activated receptor-gamma pathway activation in swine aortic valve endothelium
- PMID: 19926833
- PMCID: PMC2823293
- DOI: 10.1161/ATVBAHA.109.198549
Hypercholesterolemia induces side-specific phenotypic changes and peroxisome proliferator-activated receptor-gamma pathway activation in swine aortic valve endothelium
Abstract
Background- The endothelium of healthy aortic valves expresses different phenotypes on the aortic and ventricular sides. On the aortic side, which is susceptible to aortic valve sclerosis, there is a balanced coexpression of both propathological and protective pathways. Side-specific global gene expression can address endothelial phenotype balance in early aortic valve sclerosis.
Methods and results: Adult male swine were fed a hypercholesterolemic or an isocaloric normal diet for 2-week and 6-month periods. Hypercholesterolemia induced localized lipid insudation confined to the aortic side of the leaflet. Transcript profiling of valve endothelial populations showed that the susceptible aortic side was more sensitive to 2-week hypercholesterolemia than the ventricular side (1,325 vs 87 genes were differentially expressed). However, greater sensitivity was not evidence of a dysfunctional phenotype. Instead, pathway analyses identified differential expression of caspase 3-, peroxisome proliferator-activated receptor gamma-, TNF-alpha-, and nuclear factor-kappaB-related pathways that were consistent with a protective endothelial phenotype. This was confirmed at the protein level at 2 weeks and persisted at 6 months.
Conclusions: In a large animal model at high spatial resolution, endothelium on the pathosusceptible side of the aortic valve leaflet is responsive to hypercholesterolemia. Transcript profiles indicative of a protective phenotype were induced and persisted on the side prone to aortic valve sclerosis.
Figures



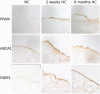
Similar articles
-
Pioglitazone attenuates valvular calcification induced by hypercholesterolemia.Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):523-32. doi: 10.1161/ATVBAHA.112.300794. Epub 2013 Jan 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23288158 Free PMC article.
-
Side-specific expression of activated leukocyte adhesion molecule (ALCAM; CD166) in pathosusceptible regions of swine aortic valve endothelium.J Heart Valve Dis. 2011 Mar;20(2):165-7. J Heart Valve Dis. 2011. PMID: 21560815 Free PMC article.
-
Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves.Circ Res. 2005 Apr 15;96(7):792-9. doi: 10.1161/01.RES.0000161998.92009.64. Epub 2005 Mar 10. Circ Res. 2005. PMID: 15761200 Free PMC article.
-
NFκB (Nuclear Factor κ-Light-Chain Enhancer of Activated B Cells) Activity Regulates Cell-Type-Specific and Context-Specific Susceptibility to Calcification in the Aortic Valve.Arterioscler Thromb Vasc Biol. 2020 Mar;40(3):638-655. doi: 10.1161/ATVBAHA.119.313248. Epub 2020 Jan 2. Arterioscler Thromb Vasc Biol. 2020. PMID: 31893948 Free PMC article.
-
Increased expression of connexin43 on the aortic valve in the hypercholesterolemic rabbit model.J Invest Surg. 2009 Mar-Apr;22(2):98-104. doi: 10.1080/08941930802713035. J Invest Surg. 2009. PMID: 19283611
Cited by
-
Animal Models for Heart Valve Research and Development.Drug Discov Today Dis Models. 2017 Summer;24:55-62. doi: 10.1016/j.ddmod.2018.04.001. Epub 2018 May 28. Drug Discov Today Dis Models. 2017. PMID: 30631375 Free PMC article.
-
Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves--association with low BMP antagonists and SMAD6.PLoS One. 2011;6(6):e20969. doi: 10.1371/journal.pone.0020969. Epub 2011 Jun 15. PLoS One. 2011. PMID: 21698246 Free PMC article.
-
The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo.Cardiovasc Res. 2013 Jul 15;99(2):315-27. doi: 10.1093/cvr/cvt101. Epub 2013 Apr 25. Cardiovasc Res. 2013. PMID: 23619421 Free PMC article. Review.
-
Involvement of Immune Cell Network in Aortic Valve Stenosis: Communication between Valvular Interstitial Cells and Immune Cells.Immune Netw. 2016 Feb;16(1):26-32. doi: 10.4110/in.2016.16.1.26. Epub 2016 Feb 25. Immune Netw. 2016. PMID: 26937229 Free PMC article. Review.
-
Models for calcific aortic valve disease in vivo and in vitro.Cell Regen. 2024 Mar 1;13(1):6. doi: 10.1186/s13619-024-00189-8. Cell Regen. 2024. PMID: 38424219 Free PMC article. Review.
References
-
- Goldbarg SH, Elmariah S, Miller MA, Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205–1213. - PubMed
-
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. - PubMed
-
- Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. A1396. - PubMed
-
- Durbin AD, Gotlieb AI. Advances towards understanding heart valve response to injury. Cardiovasc Pathol. 2002;11:69–77. - PubMed
-
- Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr., Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

