A different mechanism for the inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase by tepraloxydim
- PMID: 19926852
- PMCID: PMC2791573
- DOI: 10.1073/pnas.0908431106
A different mechanism for the inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase by tepraloxydim
Abstract
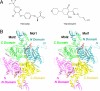
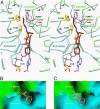
Acetyl-CoA carboxylases (ACCs) are crucial metabolic enzymes and are attractive targets for drug discovery. Haloxyfop and tepraloxydim belong to two distinct classes of commercial herbicides and kill sensitive plants by inhibiting the carboxyltransferase (CT) activity of ACC. Our earlier structural studies showed that haloxyfop is bound near the active site of the CT domain, at the interface of its dimer, and a large conformational change in the dimer interface is required for haloxyfop binding. We report here the crystal structure at 2.3 A resolution of the CT domain of yeast ACC in complex with tepraloxydim. The compound has a different mechanism of inhibiting the CT activity compared to haloxyfop, as well as the mammalian ACC inhibitor CP-640186. Tepraloxydim probes a different region of the dimer interface and requires only small but important conformational changes in the enzyme, in contrast to haloxyfop. The binding mode of tepraloxydim explains the structure-activity relationship of these inhibitors, and provides a molecular basis for their distinct sensitivity to some of the resistance mutations, as compared to haloxyfop. Despite the chemical diversity between haloxyfop and tepraloxydim, the compounds do share two binding interactions to the enzyme, which may be important anchoring points for the development of ACC inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
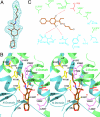
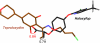
Similar articles
-
Mechanism for the inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase by pinoxaden.Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22072-7. doi: 10.1073/pnas.1012039107. Epub 2010 Dec 6. Proc Natl Acad Sci U S A. 2010. PMID: 21135213 Free PMC article.
-
Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5910-5. doi: 10.1073/pnas.0400891101. Epub 2004 Apr 12. Proc Natl Acad Sci U S A. 2004. PMID: 15079078 Free PMC article.
-
Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase.Science. 2003 Mar 28;299(5615):2064-7. doi: 10.1126/science.1081366. Science. 2003. PMID: 12663926
-
Acetyl-coenzyme A carboxylases: versatile targets for drug discovery.J Cell Biochem. 2006 Dec 15;99(6):1476-88. doi: 10.1002/jcb.21077. J Cell Biochem. 2006. PMID: 16983687 Free PMC article. Review.
-
Chemical genetics of acetyl-CoA carboxylases.Molecules. 2013 Jan 28;18(2):1704-19. doi: 10.3390/molecules18021704. Molecules. 2013. PMID: 23358327 Free PMC article. Review.
Cited by
-
Impact of a Novel W2027L Mutation and Non-Target Site Resistance on Acetyl-CoA Carboxylase-Inhibiting Herbicides in a French Lolium multiflorum Population.Genes (Basel). 2021 Nov 21;12(11):1838. doi: 10.3390/genes12111838. Genes (Basel). 2021. PMID: 34828444 Free PMC article.
-
Broad resistance to ACCase inhibiting herbicides in a ryegrass population is due only to a cysteine to arginine mutation in the target enzyme.PLoS One. 2012;7(6):e39759. doi: 10.1371/journal.pone.0039759. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768118 Free PMC article.
-
Structure and function of biotin-dependent carboxylases.Cell Mol Life Sci. 2013 Mar;70(5):863-91. doi: 10.1007/s00018-012-1096-0. Epub 2012 Aug 7. Cell Mol Life Sci. 2013. PMID: 22869039 Free PMC article. Review.
-
The enzymes of biotin dependent CO₂ metabolism: what structures reveal about their reaction mechanisms.Protein Sci. 2012 Nov;21(11):1597-619. doi: 10.1002/pro.2156. Protein Sci. 2012. PMID: 22969052 Free PMC article. Review.
-
Docking of acetyl-CoA carboxylase to the plastid envelope membrane attenuates fatty acid production in plants.Nat Commun. 2020 Dec 3;11(1):6191. doi: 10.1038/s41467-020-20014-5. Nat Commun. 2020. PMID: 33273474 Free PMC article.
References
-
- Wakil SJ, Stoops JK, Joshi VC. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. - PubMed
-
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KAH, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. - PubMed
-
- Harwood HJ., Jr Treating the metabolic syndrome: Acetyl-CoA carboxylase inhibition. Expert Opin Ther Targets. 2005;9:267–281. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

