ArfGAP1 generates an Arf1 gradient on continuous lipid membranes displaying flat and curved regions
- PMID: 19927117
- PMCID: PMC2824456
- DOI: 10.1038/emboj.2009.341
ArfGAP1 generates an Arf1 gradient on continuous lipid membranes displaying flat and curved regions
Abstract
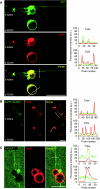
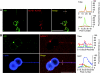
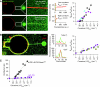
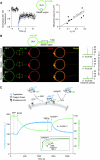
ArfGAP1, which promotes GTP hydrolysis on the small G protein Arf1 on Golgi membranes, interacts preferentially with positively curved membranes through its amphipathic lipid packing sensor (ALPS) motifs. This should influence the distribution of Arf1-GTP when flat and curved regions coexist on a continuous membrane, notably during COPI vesicle budding. To test this, we pulled tubes from giant vesicles using molecular motors or optical tweezers. Arf1-GTP distributed on the giant vesicles and on the tubes, whereas ArfGAP1 bound exclusively to the tubes. Decreasing the tube radius revealed a threshold of R approximately 35 nm for the binding of ArfGAP1 ALPS motifs. Mixing catalytic amounts of ArfGAP1 with Arf1-GTP induced a smooth Arf1 gradient along the tube. This reflects that Arf1 molecules leaving the tube on GTP hydrolysis are replaced by new Arf1-GTP molecules diffusing from the giant vesicle. The characteristic length of the gradient is two orders of magnitude larger than a COPI bud, suggesting that Arf1-GTP diffusion can readily compensate for the localized loss of Arf1 during budding and contribute to the stability of the coat until fission.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Ahmadian MR, Hoffmann U, Goody RS, Wittinghofer A (1997) Individual rate constants for the interaction of Ras proteins with GTPase-activating proteins determined by fluorescence spectroscopy. Biochemistry 36: 4535–4541 - PubMed
-
- Angelova M, Soléau S, Méléard P, Faucon F, Bothorel P (1992) Preparation of giant vesicles by external AC electric fields. Kinetics and applications. In Trends in Colloid and Interface Science VI, C Helm, M Lösche, H Möhwald (eds), pp 127–131. Steinkopff Verlag-Darmstadt, Springer-Verlag-New York: Springer Berlin/Heidelberg
-
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M (1997) N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 36: 4675–4684 - PubMed
-
- Bigay J, Gounon P, Robineau S, Antonny B (2003) Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature 426: 563–566 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

