Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin
- PMID: 19927119
- PMCID: PMC2797059
- DOI: 10.1038/emboj.2009.340
Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin
Abstract
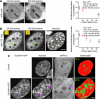
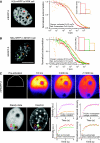
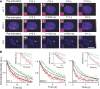
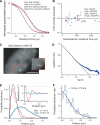
The nucleus of eukaryotes is organized into functional compartments, the two most prominent being heterochromatin and nucleoli. These structures are highly enriched in DNA, proteins or RNA, and thus thought to be crowded. In vitro, molecular crowding induces volume exclusion, hinders diffusion and enhances association, but whether these effects are relevant in vivo remains unclear. Here, we establish that volume exclusion and diffusive hindrance occur in dense nuclear compartments by probing the diffusive behaviour of inert fluorescent tracers in living cells. We also demonstrate that chromatin-interacting proteins remain transiently trapped in heterochromatin due to crowding induced enhanced affinity. The kinetic signatures of these crowding consequences allow us to derive a fractal model of chromatin organization, which explains why the dynamics of soluble nuclear proteins are affected independently of their size. This model further shows that the fractal architecture differs between heterochromatin and euchromatin, and predicts that chromatin proteins use different target-search strategies in the two compartments. We propose that fractal crowding is a fundamental principle of nuclear organization, particularly of heterochromatin maintenance.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
Fractal geometry in the nucleus.EMBO J. 2010 Jan 6;29(1):2-3. doi: 10.1038/emboj.2009.375. EMBO J. 2010. PMID: 20051993 Free PMC article. No abstract available.
References
-
- Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, Mann M (2005) Nucleolar proteome dynamics. Nature 433: 77–83 - PubMed
-
- Bancaud A, Conde e Silva N, Barbi M, Wagner G, Allemand JF, Mozziconacci J, Lavelle C, Croquette V, Victor JM, Prunell A, Viovy JL (2006) Structural plasticity of single chromatin fibers revealed by torsional manipulation. Nat Struct Mol Biol 13: 444–450 - PubMed
-
- Belmont A (2003) Dynamics of chromatin, proteins, and bodies within the cell nucleus. Curr Opin Cell Biol 15: 304–310 - PubMed
-
- Ben-Avraham S, Havlin S (2000) Diffusion and Reactions in Fractals and Disordered Systems. Cambridge: Cambridge University Press
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

