Nanoparticles and the brain: cause for concern?
- PMID: 19928180
- PMCID: PMC3804071
- DOI: 10.1166/jnn.2009.gr02
Nanoparticles and the brain: cause for concern?
Abstract
Engineered nanoparticles (NPs) are in the same size category as atmospheric ultrafine particles, < 100 nm. Per given volume, both have high numbers and surface areas compared to larger particles. The high proportion of surface atoms/molecules can give rise to a greater chemical as well as biological activity, for example the induction of reactive oxygen species in cell-free medium as well as in cells. When inhaled as singlet particles, NPs of different sizes deposit efficiently in all regions of the respiratory tract by diffusion. A major difference to larger size particles is the propensity of NPs to translocate across cell barriers from the portal of entry (e.g., the respiratory tract) to secondary organs and to enter cells by various mechanisms and associate with subcellular structures. This makes NPs uniquely suitable for therapeutic and diagnostic uses, but it also leaves target organs such as the central nervous system (CNS) vulnerable to potential adverse effects (e.g., oxidative stress). Neuronal transport of NPs has been described, involving retrograde and anterograde movement in axons and dendrites as well as perineural translocation. This is of importance for access of inhaled NPs to the CNS via sensory nerves existing in the nasopharyngeal and tracheobronchial regions of the respiratory tract. The neuronal pathway circumvents the very tight blood brain barrier. In general, translocation rates of NP from the portal of entry into the blood compartment or the CNS are very low. Important modifiers of translocation are the physicochemical characteristics of NPs, most notably their size and surface properties, particularly surface chemistry. Primary surface coating (when NPs are manufactured) and secondary surface coating (adsorption of lipids/proteins occurring at the portal of entry and during subsequent translocation) can significantly alter NP biokinetics and their effects. Implications of species differences in respiratory tract anatomy, breathing pattern and brain anatomy for extrapolation to humans of NP effects observed in rodents need to be considered. Although there are anecdotal data indicating a causal relationship between long-term ultrafine particle exposures in ambient air (e.g., traffic related) or at the workplace (e.g., metal fumes) and resultant neurotoxic effects in humans, more studies are needed to test the hypothesis that inhaled nanoparticles cause neurodegenerative effects. Some but probably not the majority of NPs will have a significant toxicity (hazard) potential, and this will pose a significant risk if there is a sufficient exposure. The challenge is to identify such hazardous NPs and take appropriate measures to prevent exposure.
Figures





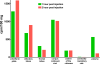
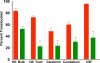
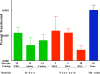
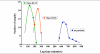
 ) or as solution (
) or as solution ( ) during 6 hrs following intranasal instillation in rats. (redrawn from results by Zhang et al., 2006).
) during 6 hrs following intranasal instillation in rats. (redrawn from results by Zhang et al., 2006).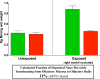





Similar articles
-
Translocation of inhaled ultrafine particles to the brain.Inhal Toxicol. 2004 Jun;16(6-7):437-45. doi: 10.1080/08958370490439597. Inhal Toxicol. 2004. PMID: 15204759
-
Differences in the biokinetics of inhaled nano- versus micrometer-sized particles.Acc Chem Res. 2013 Mar 19;46(3):714-22. doi: 10.1021/ar300043r. Epub 2012 Sep 17. Acc Chem Res. 2013. PMID: 22980029 Free PMC article.
-
Discovery of unique and ENM- specific pathophysiologic pathways: Comparison of the translocation of inhaled iridium nanoparticles from nasal epithelium versus alveolar epithelium towards the brain of rats.Toxicol Appl Pharmacol. 2016 May 15;299:41-6. doi: 10.1016/j.taap.2016.02.004. Epub 2016 Feb 6. Toxicol Appl Pharmacol. 2016. PMID: 26861261 Free PMC article.
-
Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions.IEEE Trans Nanobioscience. 2007 Dec;6(4):331-40. doi: 10.1109/tnb.2007.909005. IEEE Trans Nanobioscience. 2007. PMID: 18217626 Review.
-
Risks from accidental exposures to engineered nanoparticles and neurological health effects: a critical review.Part Fibre Toxicol. 2010 Dec 21;7:42. doi: 10.1186/1743-8977-7-42. Part Fibre Toxicol. 2010. PMID: 21176150 Free PMC article. Review.
Cited by
-
Within-city Spatial Variations in Ambient Ultrafine Particle Concentrations and Incident Brain Tumors in Adults.Epidemiology. 2020 Mar;31(2):177-183. doi: 10.1097/EDE.0000000000001137. Epidemiology. 2020. PMID: 31714401 Free PMC article.
-
Synergistic effects of engineered nanoparticles and organics released from laser printers using nano-enabled toners: potential health implications from exposures to the emitted organic aerosol.Environ Sci Nano. 2017 Nov 1;4(11):2144-2156. doi: 10.1039/C7EN00573C. Epub 2017 Aug 30. Environ Sci Nano. 2017. PMID: 30197786 Free PMC article.
-
INSIdE NANO: a systems biology framework to contextualize the mechanism-of-action of engineered nanomaterials.Sci Rep. 2019 Jan 17;9(1):179. doi: 10.1038/s41598-018-37411-y. Sci Rep. 2019. PMID: 30655578 Free PMC article.
-
Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment.Nanomaterials (Basel). 2022 May 25;12(11):1810. doi: 10.3390/nano12111810. Nanomaterials (Basel). 2022. PMID: 35683670 Free PMC article. Review.
-
Nanotechnology-based Nose-to-brain Delivery in Epilepsy: A NovelApproach to Diagnosis and Treatment.Pharm Nanotechnol. 2024;12(4):314-328. doi: 10.2174/0122117385265554230919070402. Pharm Nanotechnol. 2024. PMID: 37818558 Review.
References
-
- Adams RJ, Bray D. Rapid transport of foreign particles microinjected into crab axons. Nature. 1983;303:718–720. - PubMed
-
- Asgharian B, Miller F, Subramaniam RP. Dosimetry software to predict particle deposition in humans and rats. CIIT Activities. 1999;19(3)
-
- Ball P. Nanoparticles in sun creams can stress brain cells. Nature (News) 2006 doi: 10.1038; published on line June 16, 2006 ( http://www.nature.com/news/2006/060616/full/news060612-14.html)
-
- Barrios FA, Gonzalez L, Favila R, Alonso ME, Salgado PM, Diaz R, Fernandez-Ruiz J. Olfaction and neurodegeneration in HD. Neuroreport. 2007;18(1):73–76. - PubMed
-
- Bodian D, Howe HA. Experimental studies on intraneural spread of poliomyelitis virus. Bull. Johns Hopkins Hosp. 1941;69:248–267.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
