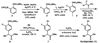
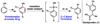Suzuki-Miyaura coupling of aryl carbamates, carbonates, and sulfamates
- PMID: 19928764
- PMCID: PMC2801021
- DOI: 10.1021/ja906477r
Suzuki-Miyaura coupling of aryl carbamates, carbonates, and sulfamates
Abstract
The first Suzuki-Miyaura cross-couplings of carbamates, carbonates, and sulfamates is described. The method provides a powerful means of using simple derivatives of phenol as precursors to polysubstituted aromatic compounds, as exemplified by a concise synthesis of the anti-inflammatory drug flurbiprofen.
Figures
Similar articles
-
Rapid nickel-catalyzed Suzuki-Miyaura cross-couplings of aryl carbamates and sulfamates utilizing microwave heating.J Org Chem. 2011 Mar 4;76(5):1507-10. doi: 10.1021/jo1024464. Epub 2011 Jan 20. J Org Chem. 2011. PMID: 21250707
-
Suzuki-Miyaura cross-coupling of aryl carbamates and sulfamates: experimental and computational studies.J Am Chem Soc. 2011 Apr 27;133(16):6352-63. doi: 10.1021/ja200398c. Epub 2011 Apr 1. J Am Chem Soc. 2011. PMID: 21456551 Free PMC article.
-
Nickel-catalyzed amination of aryl sulfamates and carbamates using an air-stable precatalyst.Org Lett. 2012 Aug 17;14(16):4182-5. doi: 10.1021/ol301847m. Epub 2012 Jul 31. Org Lett. 2012. PMID: 22849697 Free PMC article.
-
Directed reactions of organocopper reagents.Chem Rev. 2008 Aug;108(8):2928-51. doi: 10.1021/cr078352c. Chem Rev. 2008. PMID: 18698734 Review. No abstract available.
-
Catalytic C-H amination: recent progress and future directions.Chem Commun (Camb). 2009 Sep 14;(34):5061-74. doi: 10.1039/b905820f. Epub 2009 Jul 7. Chem Commun (Camb). 2009. PMID: 20448953 Review.
Cited by
-
The Pyridyldiisopropylsilyl Group: A Masked Functionality and Directing Group for Monoselective ortho-Acyloxylation and ortho-Halogenation Reactions of Arenes.Adv Synth Catal. 2011 May 1;353(8):10.1002/adsc.201000975. doi: 10.1002/adsc.201000975. Adv Synth Catal. 2011. PMID: 24363640 Free PMC article.
-
The Versatile and Strategic O-Carbamate Directed Metalation Group in the Synthesis of Aromatic Molecules: An Update.Chem Rev. 2024 Jun 26;124(12):7731-7828. doi: 10.1021/acs.chemrev.3c00923. Epub 2024 Jun 12. Chem Rev. 2024. PMID: 38864673 Free PMC article. Review.
-
Mechanistic Study of an Improved Ni Precatalyst for Suzuki-Miyaura Reactions of Aryl Sulfamates: Understanding the Role of Ni(I) Species.J Am Chem Soc. 2017 Jan 18;139(2):922-936. doi: 10.1021/jacs.6b11412. Epub 2017 Jan 10. J Am Chem Soc. 2017. PMID: 28009513 Free PMC article.
-
N-Heterocyclic Carbene Bound Nickel(I) Complexes and Their Roles in Catalysis.Organometallics. 2011 May 9;30(9):2546-2552. doi: 10.1021/om200090d. Organometallics. 2011. PMID: 21572533 Free PMC article.
-
Nickel-catalyzed amination of aryl carbamates and sequential site-selective cross-couplings.Chem Sci. 2011 Sep 1;2(9):1766-1771. doi: 10.1039/c1sc00230a. Epub 2011 Jun 9. Chem Sci. 2011. PMID: 31105924 Free PMC article.
References
-
- Diederich F, Meijere A, editors. Metal-Catalyzed Cross-Coupling Reactions. Weinheim: Wiley-VCH; 2004.
- Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359–1469. - PubMed
- Miyaura N, editor. Topics in Current Chemistry. Vol. 219. New York: Springer-Verlag; 2002.
- Corbet J, Mignani G. Chem. Rev. 2006;106:2651–2710. - PubMed
- Negishi E. Bull. Chem. Soc. Jpn. 2007;80:233–257.
-
- Tobisu M, Shimasaki T, Chatani N. Angew. Chem. Int. Ed. 2008;47:4866–4869. - PubMed
-
-
For reviews, see: Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2009;48:3565–3568.Gooβen LJ, Gooβen K, Stanciu C. Angew. Chem. Int. Ed. 2009;48:3569–3571.Knochel P, Gavryushin A. Synfacts. 2009;2:200..
-
-
-
The ortho-directing ability of methyl ethers is modest (see ref and refs therein), whereas ortho substitution of aryl pivalates presents a considerable challenge.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases