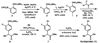
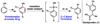Suzuki-Miyaura coupling of aryl carbamates, carbonates, and sulfamates
- PMID: 19928764
- PMCID: PMC2801021
- DOI: 10.1021/ja906477r
Suzuki-Miyaura coupling of aryl carbamates, carbonates, and sulfamates
Abstract
The first Suzuki-Miyaura cross-couplings of carbamates, carbonates, and sulfamates is described. The method provides a powerful means of using simple derivatives of phenol as precursors to polysubstituted aromatic compounds, as exemplified by a concise synthesis of the anti-inflammatory drug flurbiprofen.
Figures
References
-
- Diederich F, Meijere A, editors. Metal-Catalyzed Cross-Coupling Reactions. Weinheim: Wiley-VCH; 2004.
- Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359–1469. - PubMed
- Miyaura N, editor. Topics in Current Chemistry. Vol. 219. New York: Springer-Verlag; 2002.
- Corbet J, Mignani G. Chem. Rev. 2006;106:2651–2710. - PubMed
- Negishi E. Bull. Chem. Soc. Jpn. 2007;80:233–257.
-
- Tobisu M, Shimasaki T, Chatani N. Angew. Chem. Int. Ed. 2008;47:4866–4869. - PubMed
-
-
For reviews, see: Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2009;48:3565–3568.Gooβen LJ, Gooβen K, Stanciu C. Angew. Chem. Int. Ed. 2009;48:3569–3571.Knochel P, Gavryushin A. Synfacts. 2009;2:200..
-
-
-
The ortho-directing ability of methyl ethers is modest (see ref and refs therein), whereas ortho substitution of aryl pivalates presents a considerable challenge.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases