Identification of novel non-hydroxamate anthrax toxin lethal factor inhibitors by topomeric searching, docking and scoring, and in vitro screening
- PMID: 19928768
- PMCID: PMC2805240
- DOI: 10.1021/ci900186w
Identification of novel non-hydroxamate anthrax toxin lethal factor inhibitors by topomeric searching, docking and scoring, and in vitro screening
Abstract
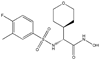
Anthrax is an infectious disease caused by Bacillus anthracis, a Gram-positive, rod-shaped, anaerobic bacterium. The lethal factor (LF) enzyme is secreted by B. anthracis as part of a tripartite exotoxin and is chiefly responsible for anthrax-related cytotoxicity. As LF can remain in the system long after antibiotics have eradicated B. anthracis from the body, the preferred therapeutic modality would be the administration of antibiotics together with an effective LF inhibitor. Although LF has garnered a great deal of attention as an attractive target for rational drug design, relatively few published inhibitors have demonstrated activity in cell-based assays and, to date, no LF inhibitor is available as a therapeutic or preventive agent. Here we present a novel in silico high-throughput virtual screening protocol that successfully identified 5 non-hydroxamic acid small molecules as new, preliminary LF inhibitor scaffolds with low micromolar inhibition against that target, resulting in a 12.8% experimental hit rate. This protocol screened approximately 35 million nonredundant compounds for potential activity against LF and comprised topomeric searching, docking and scoring, and drug-like filtering. Among these 5 hit compounds, none of which has previously been identified as a LF inhibitor, three exhibited experimental IC(50) values less than 100 microM. These three preliminary hits may potentially serve as scaffolds for lead optimization as well as templates for probe compounds to be used in mechanistic studies. Notably, our docking simulations predicted that these novel hits are likely to engage in critical ligand-receptor interactions with nearby residues in at least two of the three (S1', S1-S2, and S2') subsites in the LF substrate binding area. Further experimental characterization of these compounds is in process. We found that micromolar-level LF inhibition can be attained by compounds with non-hydroxamate zinc-binding groups that exhibit monodentate zinc chelation as long as key hydrophobic interactions with at least two LF subsites are retained.
Figures





Similar articles
-
Inhibitors of the Metalloproteinase Anthrax Lethal Factor.Curr Top Med Chem. 2016;16(21):2350-8. doi: 10.2174/1568026616666160413135732. Curr Top Med Chem. 2016. PMID: 27072692 Free PMC article. Review.
-
Statistical analysis, optimization, and prioritization of virtual screening parameters for zinc enzymes including the anthrax toxin lethal factor.Curr Top Med Chem. 2014;14(18):2105-14. doi: 10.2174/1568026614666141106163011. Curr Top Med Chem. 2014. PMID: 25373478 Free PMC article. Review.
-
Probing the S2' Subsite of the Anthrax Toxin Lethal Factor Using Novel N-Alkylated Hydroxamates.J Med Chem. 2015 Nov 12;58(21):8723-33. doi: 10.1021/acs.jmedchem.5b01446. Epub 2015 Oct 29. J Med Chem. 2015. PMID: 26492514
-
Development of a comprehensive, validated pharmacophore hypothesis for anthrax toxin lethal factor (LF) inhibitors using genetic algorithms, Pareto scoring, and structural biology.J Chem Inf Model. 2012 Jul 23;52(7):1886-97. doi: 10.1021/ci300121p. Epub 2012 Jun 25. J Chem Inf Model. 2012. PMID: 22697455 Free PMC article.
-
Insilico studies on anthrax lethal factor inhibitors: pharmacophore modeling and virtual screening approaches towards designing of novel inhibitors for a killer.J Mol Graph Model. 2010 Sep;29(2):256-65. doi: 10.1016/j.jmgm.2010.07.002. Epub 2010 Jul 15. J Mol Graph Model. 2010. PMID: 20727800
Cited by
-
Inhibitors of the Metalloproteinase Anthrax Lethal Factor.Curr Top Med Chem. 2016;16(21):2350-8. doi: 10.2174/1568026616666160413135732. Curr Top Med Chem. 2016. PMID: 27072692 Free PMC article. Review.
-
Small molecule inhibitors of anthrax lethal factor toxin.Bioorg Med Chem. 2014 Jan 1;22(1):419-34. doi: 10.1016/j.bmc.2013.11.009. Epub 2013 Nov 13. Bioorg Med Chem. 2014. PMID: 24290062 Free PMC article.
-
Statistical analysis, optimization, and prioritization of virtual screening parameters for zinc enzymes including the anthrax toxin lethal factor.Curr Top Med Chem. 2014;14(18):2105-14. doi: 10.2174/1568026614666141106163011. Curr Top Med Chem. 2014. PMID: 25373478 Free PMC article. Review.
-
Electrostatically Embedded Many-Body Expansion for Neutral and Charged Metalloenzyme Model Systems.J Chem Theory Comput. 2012 Jan 10;8(1):1-5. doi: 10.1021/ct200637v. Epub 2011 Nov 29. J Chem Theory Comput. 2012. PMID: 22639556 Free PMC article.
-
Ligand-induced expansion of the S1' site in the anthrax toxin lethal factor.FEBS Lett. 2015 Dec 21;589(24 Pt B):3836-41. doi: 10.1016/j.febslet.2015.11.005. Epub 2015 Nov 11. FEBS Lett. 2015. PMID: 26578066 Free PMC article.
References
-
- Chopra A, Boone S, Liang X, Duesbery N. Anthrax lethal factor proteolysis and inactivation of MAPK kinase. J. Biol. Chem. 2003;278:9402–9406. - PubMed
-
- Turk B. Discovery and development of anthrax lethal factor metalloproteinase inhibitors. Curr. Pharm. Biotechnol. 2008;9:24–33. - PubMed
-
- Gaddis B, Avramova L, Chmielewski J. Inhibitors of anthrax lethal factor. Bioorg. Med. Chem. Lett. 2007;17:4575–4578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

