The biochemical basis for stereochemical control in polyketide biosynthesis
- PMID: 19928853
- PMCID: PMC3699857
- DOI: 10.1021/ja908296m
The biochemical basis for stereochemical control in polyketide biosynthesis
Abstract
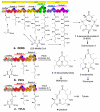
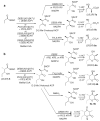
One of the most striking features of complex polyketides is the presence of numerous methyl- and hydroxyl-bearing stereogenic centers. To investigate the biochemical basis for the control of polyketide stereochemistry and to establish the timing and mechanism of the epimerization at methyl-bearing centers, a series of incubations was carried out using reconstituted components from a variety of modular polyketide synthases. In all cases the stereochemistry of the product was directly correlated with the intrinsic stereospecificity of the ketoreductase domain, independent of the particular chain elongation domains that were used, thereby establishing that methyl group epimerization, when it does occur, takes place after ketosynthase-catalyzed chain elongation. The finding that there were only minor differences in the rates of product formation observed for parallel incubations using an epimerizing ketoreductase domain and the nonepimerizing ketoreductase domain supports the proposal that the epimerization is catalyzed by the ketoreductase domain itself.
Figures


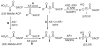

References
-
- Khosla C, Tang Y, Chen AY, Schnarr NA, Cane DE. Annu. Rev. Biochem. 2007;76:195–221. - PubMed
- Khosla C, Kapur S, Cane DE. Curr. Opin. Chem. Biol. 2009;13:135–143. - PMC - PubMed
- Hopwood DA, editor. Methods in Enzymology. Complex Enzymes in Microbial Natural Product Biosynthesis, Part B: Polyketides, Aminocoumarins and Carbohydrates. Vol. 459. Academic Press; 2009. Vol. 459. - PubMed
-
- Kao CM, McPherson M, McDaniel RN, Fu H, Cane DE, Khosla C. J. Am. Chem. Soc. 1998;120:2478–2479.
-
- McPherson M, Khosla C, Cane DE. J. Am. Chem. Soc. 1998;120:3267–3268.
- Yin Y, Gokhale R, Khosla C, Cane DE. Bioorg. Med. Chem. Lett. 2001;11:1477–1479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

