Direct observation of a photoinduced nonstabilized nitrile imine structure in the solid state
- PMID: 19928921
- PMCID: PMC2797338
- DOI: 10.1021/ja9094523
Direct observation of a photoinduced nonstabilized nitrile imine structure in the solid state
Abstract
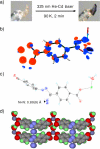
We report the direct observation of a bent geometry for a nonstabilized nitrile imine in a metal-coordination crystal. The photoinduced tetrazole ring rupture to release N(2) appears to depend on the size of voids around the N(3)-N(4) bond in the crystal lattice. We further observed the selective formation of the 1,3-addition product when a reactive nitrile imine was photogenerated in water. Overall, the bent nitrile imine geometry agrees with the 1,3-dipolar structure, a transient reactive species that mediates the photoinduced 1,3-dipolar cycloaddition in the aqueous medium.
Figures




References
-
- Clovis JS, Eckell A, Huisgen R, Sustmann R. Chem. Ber. 1967;100:60–70.
-
- Padwa A, Nahm S, Sato E. J. Org. Chem. 1978;43:1664–1671.
- Meier H, Heimgartner H. Helv. Chim. Acta. 1985;68:1283–1300.
-
-
For an excellent review, see: Bertrand G, Wentrup C. Angew. Chem. Int. Ed. 1994;33:527–545.; and references therein.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

