Structure, stability, and interaction of the fibrin(ogen) alphaC-domains
- PMID: 19928926
- PMCID: PMC2812052
- DOI: 10.1021/bi901640e
Structure, stability, and interaction of the fibrin(ogen) alphaC-domains
Abstract
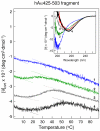
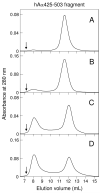
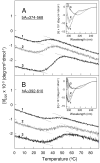
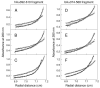
Our recent study established the NMR structure of the recombinant bAalpha406-483 fragment corresponding to the NH(2)-terminal half of the bovine fibrinogen alphaC-domain and revealed that at increasing concentrations this fragment forms oligomers (self-associates). The major goals of the study presented here were to determine the structure and self-association of the full-length human fibrinogen alphaC-domains. To accomplish these goals, we prepared a recombinant human fragment, hAalpha425-503, homologous to bovine bAalpha406-483, and demonstrated using NMR, CD, and size-exclusion chromatography that its overall fold and ability to form oligomers are similar to those of bAalpha406-483. We also prepared recombinant hAalpha392-610 and bAalpha374-568 fragments corresponding to the full-length human and bovine alphaC-domains, respectively, and tested their structure, stability, and ability to self-associate. Size-exclusion chromatography revealed that both fragments form reversible oligomers in a concentration-dependent manner. Their oligomerization was confirmed in sedimentation equilibrium experiments, which also established the self-association affinities of these fragments and revealed that the addition of each monomer to assembling alphaC-oligomers substantially increases the stabilizing free energy. In agreement, unfolding experiments monitored by CD established that self-association of both fragments results in a significant increase in their thermal stability. Analysis of CD spectra of both fragments revealed that alphaC self-association results in an increase in the level of regular structure, implying that the COOH-terminal half of the alphaC-domain adopts an ordered conformation in alphaC-oligomers and that this domain contains two independently folded subdomains. Altogether, these data further clarify the structure of the human and bovine alphaC-domains and the molecular mechanism of their self-association into alphaC-polymers in fibrin.
Figures



References
-
- Henschen A, McDonagh J. Fibrinogen, fibrin and factor XIII. In: Zwaal RFA, Hemker HC, editors. Blood Coagulation. Elsievier Science Publishers; Amsterdam: 1986. pp. 171–241.
-
- Medved LV, Gorkun OV, Privalov PL. Structural organization of C-terminal parts of fibrinogen Aα-chains. FEBS Lett. 1983;160:291–295. - PubMed
-
- Erickson HP, Fowler WE. Electron microscopy of fibrinogen, its plasmic fragments and small polymers. Ann. N. Y. Acad. Sci. 1983;408:146–163. - PubMed
-
- Weisel JW, Medved L. The structure and function of the αC domains of fibrinogen. Ann. N. Y. Acad. Sci. 2001;936:312–327. - PubMed
-
- Medved LV, Gorkun OV, Manyakov VF, Belitser VA. The role of fibrinogen αC-domains in the fibrin assembly process. FEBS Lett. 1985;181:109–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

