Antiviral activity of the interferon-induced cellular protein BST-2/tetherin
- PMID: 19929170
- PMCID: PMC2858902
- DOI: 10.1089/aid.2009.0253
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin
Abstract
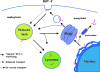
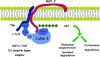
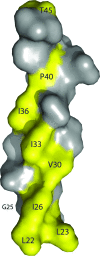
Pathogenic microorganisms encode proteins that antagonize specific aspects of innate or adaptive immunity. Just as the study of the HIV-1 accessory protein Vif led to the identification of cellular cytidine deaminases as host defense proteins, the study of HIV-1 Vpu recently led to the discovery of the interferon-induced transmembrane protein BST-2 (CD317; tetherin) as a novel component of the innate defense against enveloped viruses. BST-2 is an unusually structured protein that restricts the release of fully formed progeny virions from infected cells, presumably by a direct retention mechanism that is independent of any viral protein target. Its spectrum of activity includes at least four virus families: retroviruses, filoviruses, arenaviruses, and herpesviruses. Viral antagonists of BST-2 include HIV-1 Vpu, HIV-2 and SIV Env, SIV Nef, the Ebola envelope glycoprotein, and the K5 protein of KSHV. The mechanisms of antagonism are diverse and currently include viral cooption of cellular endosomal trafficking and protein degradation pathways, including those mediated by ubiquitination. Orthologs of human BST-2 are present in mammals. Primate BST-2 proteins are differentially sensitive to antagonism by lentiviral Vpu and Nef proteins, suggesting that BST-2 has subjected lentiviruses to evolutionary pressure and presents barriers to cross-species transmission. BST-2 functions not only as an effector of the interferon-induced antiviral response but also as a negative feedback regulator of interferon production by plasmacytoid dendritic cells. Future work will focus on the role and regulation of BST-2 during the innate response to viral infection, on the mechanisms of restriction and of antagonism by viral gene products, and on the role of BST-2 in primate lentiviral evolution. The augmentation of BST-2 activity and the inhibition of virally encoded antagonists, in particular Vpu, represent new approaches to the prevention and treatment of HIV-1 infection.
Figures



References
-
- Strebel K. Klimkait T. Martin MA. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241:1221–1223. - PubMed
-
- Cohen EA. Terwilliger EF. Sodroski JG. Haseltine WA. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988;334:532–534. - PubMed
-
- Sakai H. Tokunaga K. Kawamura M. Adachi A. Function of human immunodeficiency virus type 1 Vpu protein in various cell types. J Gen Virol. 1995;76(Pt 11):2717–2722. - PubMed
-
- Yasuda Y. Miyake S. Kato S, et al. Interferon-alpha treatment leads to accumulation of virus particles on the surface of cells persistently infected with the human immunodeficiency virus type 1. J Acquir Immune Defic Syndr. 1990;3(11):1046–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

