Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation
- PMID: 19929420
- PMCID: PMC2793528
- DOI: 10.1667/RR1617.1
Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation
Abstract
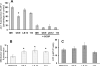
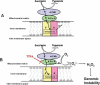
Ionizing radiation induces chronic metabolic oxidative stress and a mutator phenotype in hamster fibroblasts that is mediated by H(2)O(2), but the intracellular source of H(2)O(2) is not well defined. To determine the role of mitochondria in the radiation-induced mutator phenotype, end points of mitochondrial function were determined in unstable (CS-9 and LS-12) and stable (114) hamster fibroblast cell lines derived from GM10115 cells exposed to 10 Gy X rays. Cell lines isolated after irradiation demonstrated a 20-40% loss of mitochondrial membrane potential and an increase in mitochondrial content compared to the parental cell line GM10115. Surprisingly, no differences were observed in steady-state levels of ATP (P > 0.05). Unstable clones demonstrated increased oxygen consumption (two- to threefold; CS-9) and/or increased mitochondrial electron transport chain (ETC) complex II activity (twofold; LS-12). Using Western blot analysis and Blue Native gel electrophoresis, a significant increase in complex II subunit B protein levels was observed in LS-12 cells. Furthermore, immunoprecipitation assays revealed evidence of abnormal complex II assembly in LS-12 cells. Treatment of LS-12 cells with an inhibitor of ETC complex II (thenoyltrifluoroacetone) resulted in significant decreases in the steady-state levels of H(2)O(2) and a 50% reduction in mutation frequency as well as a 16% reduction in CAD gene amplification frequency. These data show that radiation-induced genomic instability was accompanied by evidence of mitochondrial dysfunction leading to increased steady-state levels of H(2)O(2) that contributed to increased mutation frequency and gene amplification. These results support the hypothesis that mitochondrial dysfunction originating from complex II can contribute to radiation-induced genomic instability by increasing steady-state levels of reactive oxygen species.
Figures


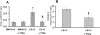
References
-
- Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. - PubMed
-
- Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: A review. Mutagenesis. 2006;21:361–367. - PubMed
-
- Miller JH, Jin S, Morgan WF, Yang A, Wan Y, Aypar U, Peters JS, Springer DL. Profiling mitochondrial proteins in radiation-induced genome-unstable cell lines with persistent oxidative stress by mass spectrometry. Radiat. Res. 2008;169:700–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

