Origin matters: differences in embryonic tissue origin and Wnt signaling determine the osteogenic potential and healing capacity of frontal and parietal calvarial bones
- PMID: 19929441
- PMCID: PMC3154006
- DOI: 10.1359/jbmr.091116
Origin matters: differences in embryonic tissue origin and Wnt signaling determine the osteogenic potential and healing capacity of frontal and parietal calvarial bones
Abstract
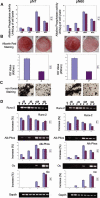
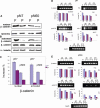
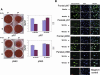
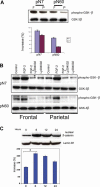
Calvarial bones arise from two embryonic tissues, namely, the neural crest and the mesoderm. In this study we have addressed the important question of whether disparate embryonic tissue origins impart variable osteogenic potential and regenerative capacity to calvarial bones, as well as what the underlying molecular mechanism(s). Thus, by performing in vitro and in vivo studies, we have investigated whether differences exist between neural crest-derived frontal and paraxial mesodermal-derived parietal bone. Of interest, our data indicate that calvarial bone osteoblasts of neural crest origin have superior potential for osteogenic differentiation. Furthermore, neural crest-derived frontal bone displays a superior capacity to undergo osseous healing compared with calvarial bone of paraxial mesoderm origin. Our study identified both in vitro and in vivo enhanced endogenous canonical Wnt signaling in frontal bone compared with parietal bone. In addition, we demonstrate that constitutive activation of canonical Wnt signaling in paraxial mesodermal-derived parietal osteoblasts mimics the osteogenic potential of frontal osteoblasts, whereas knockdown of canonical Wnt signaling dramatically impairs the greater osteogenic potential of neural crest-derived frontal osteoblasts. Moreover, fibroblast growth factor 2 (FGF-2) treatment induces phosphorylation of GSK-3beta and increases the nuclear levels of beta-catenin in osteoblasts, suggesting that enhanced activation of Wnt signaling might be mediated by FGF. Taken together, our data provide compelling evidence that indeed embryonic tissue origin makes a difference and that active canonical Wnt signaling plays a major role in contributing to the superior intrinsic osteogenic potential and tissue regeneration observed in neural crest-derived frontal bone.
2010 American Society for Bone and Mineral Research.
Figures



References
-
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. - PubMed
-
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. - PubMed
-
- LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

