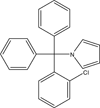
Formulation-based approach to support early drug discovery and development efforts: a case study with enteric microencapsulation dosage form development for a triarylmethane derivative TRAM-34; a novel potential immunosuppressant
- PMID: 19929567
- PMCID: PMC3337761
- DOI: 10.3109/03639040903329554
Formulation-based approach to support early drug discovery and development efforts: a case study with enteric microencapsulation dosage form development for a triarylmethane derivative TRAM-34; a novel potential immunosuppressant
Abstract
Background: Enteric microencapsulation of the potential immunosuppressant TRAM-34 was investigated as a means of enhancing oral drug delivery and minimizing or eliminating hydrolysis of pyrazole-substituted triarylmethane to the respective alcohol.
Method: TRAM-34 was successfully enteric microencapsulated by a coacervation method using the pH-sensitive Eudragit L 100 polymer. In this study, we utilized water-miscible organic solvents such as acetone and ethanol, which are considered safe class 3 solvents according to the ICH guideline. We deemed such an approach suitable for safe scale up and for enteric coating application to other compounds of a similar lipophilicity.
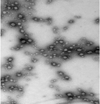
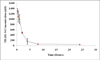
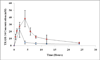
Results: The resulting microparticles were spherical and uniform with an average particle size of 460 microm at 15% theoretical loading. The encapsulation efficiency was 90 +/- 1.9% and the percentage yield was found to be 91.5 +/- 0.3%. The oral administration in rhesus macaques of TRAM-34-loaded enteric-coated microparticles illustrated six times enhancement in its oral bioavailability. However, the TRAM-34 plasma concentration was less than the therapeutic effective level.
Conclusion: The low oral bioavailability, even after enteric coating, could be attributed to the compound's inherent absorption characteristics and high lipophilicity.
Figures


Similar articles
-
Encapsulation of lipophilic drugs within enteric microparticles by a novel coacervation method.Int J Pharm. 2006 Dec 1;326(1-2):128-38. doi: 10.1016/j.ijpharm.2006.07.013. Epub 2006 Jul 15. Int J Pharm. 2006. PMID: 16942845
-
Encapsulation of poorly soluble basic drugs into enteric microparticles: a novel approach to enhance their oral bioavailability.Int J Pharm. 2011 Sep 15;416(1):55-60. doi: 10.1016/j.ijpharm.2011.05.079. Epub 2011 Jun 14. Int J Pharm. 2011. PMID: 21679756
-
Preparation and evaluation of posaconazole-loaded enteric microparticles in rats.Drug Dev Ind Pharm. 2017 Apr;43(4):618-627. doi: 10.1080/03639045.2016.1275667. Epub 2017 Jan 8. Drug Dev Ind Pharm. 2017. PMID: 28005452
-
Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability.Eur J Pharm Sci. 2019 Oct 1;138:105019. doi: 10.1016/j.ejps.2019.105019. Epub 2019 Jul 30. Eur J Pharm Sci. 2019. PMID: 31374253 Review.
-
A Review on Enteric Coated Pellets Composed of Core Pellets Prepared by Extrusion-Spheronization.Recent Pat Drug Deliv Formul. 2019;13(2):83-90. doi: 10.2174/1872211313666190212115139. Recent Pat Drug Deliv Formul. 2019. PMID: 30747090 Review.
Cited by
-
KCa3.1 Channel Modulators as Potential Therapeutic Compounds for Glioblastoma.Curr Neuropharmacol. 2018;16(5):618-626. doi: 10.2174/1570159X15666170630164226. Curr Neuropharmacol. 2018. PMID: 28676010 Free PMC article.
-
Repurposing the KCa3.1 inhibitor senicapoc for Alzheimer's disease.Ann Clin Transl Neurol. 2019 Mar 18;6(4):723-738. doi: 10.1002/acn3.754. eCollection 2019 Apr. Ann Clin Transl Neurol. 2019. PMID: 31019997 Free PMC article.
-
Endothelial KCa channels: Novel targets to reduce atherosclerosis-driven vascular dysfunction.Front Pharmacol. 2023 Mar 31;14:1151244. doi: 10.3389/fphar.2023.1151244. eCollection 2023. Front Pharmacol. 2023. PMID: 37063294 Free PMC article. Review.
-
IK Channel-Independent Effects of Clotrimazole and Senicapoc on Cancer Cells Viability and Migration.Int J Mol Sci. 2023 Nov 14;24(22):16285. doi: 10.3390/ijms242216285. Int J Mol Sci. 2023. PMID: 38003471 Free PMC article.
-
Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons.Nat Commun. 2013;4:1465. doi: 10.1038/ncomms2478. Nat Commun. 2013. PMID: 23403566
References
-
- Amorim MJ, Ferreira JP. Microparticles for delivering therapeutic peptides and proteins to the lumen of the small intestine. Eur. J. Pharm. Biopharm. 2001;52:39–44. - PubMed
-
- Ashford M, Fell JT. Targeting drugs to the colon: delivery systems for oral administration. J. Drug. Target. 1994;2:241–257. - PubMed
-
- Barton AFM. CRC Handbook of Solubility Parameters and Other Cohesion Parameters. Florida, USA: CRC Press Inc.; 1983.
-
- Burgess DJ. Injectable dispersed systems: formulation, processing, and performance. Boca Raton, USA: Taylor & Francis; 2005.
-
- Caldwell GW, Ritchie DM, Masucci JA, Hageman W, Yan Z. The new pre- preclinical paradigm: compound optimization in early and late phase drug discovery. Curr. Top. Med. Chem. 2001;1:353–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
