Formulation-based approach to support early drug discovery and development efforts: a case study with enteric microencapsulation dosage form development for a triarylmethane derivative TRAM-34; a novel potential immunosuppressant
- PMID: 19929567
- PMCID: PMC3337761
- DOI: 10.3109/03639040903329554
Formulation-based approach to support early drug discovery and development efforts: a case study with enteric microencapsulation dosage form development for a triarylmethane derivative TRAM-34; a novel potential immunosuppressant
Abstract
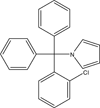
Background: Enteric microencapsulation of the potential immunosuppressant TRAM-34 was investigated as a means of enhancing oral drug delivery and minimizing or eliminating hydrolysis of pyrazole-substituted triarylmethane to the respective alcohol.
Method: TRAM-34 was successfully enteric microencapsulated by a coacervation method using the pH-sensitive Eudragit L 100 polymer. In this study, we utilized water-miscible organic solvents such as acetone and ethanol, which are considered safe class 3 solvents according to the ICH guideline. We deemed such an approach suitable for safe scale up and for enteric coating application to other compounds of a similar lipophilicity.
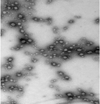
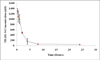
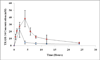
Results: The resulting microparticles were spherical and uniform with an average particle size of 460 microm at 15% theoretical loading. The encapsulation efficiency was 90 +/- 1.9% and the percentage yield was found to be 91.5 +/- 0.3%. The oral administration in rhesus macaques of TRAM-34-loaded enteric-coated microparticles illustrated six times enhancement in its oral bioavailability. However, the TRAM-34 plasma concentration was less than the therapeutic effective level.
Conclusion: The low oral bioavailability, even after enteric coating, could be attributed to the compound's inherent absorption characteristics and high lipophilicity.
Figures


References
-
- Amorim MJ, Ferreira JP. Microparticles for delivering therapeutic peptides and proteins to the lumen of the small intestine. Eur. J. Pharm. Biopharm. 2001;52:39–44. - PubMed
-
- Ashford M, Fell JT. Targeting drugs to the colon: delivery systems for oral administration. J. Drug. Target. 1994;2:241–257. - PubMed
-
- Barton AFM. CRC Handbook of Solubility Parameters and Other Cohesion Parameters. Florida, USA: CRC Press Inc.; 1983.
-
- Burgess DJ. Injectable dispersed systems: formulation, processing, and performance. Boca Raton, USA: Taylor & Francis; 2005.
-
- Caldwell GW, Ritchie DM, Masucci JA, Hageman W, Yan Z. The new pre- preclinical paradigm: compound optimization in early and late phase drug discovery. Curr. Top. Med. Chem. 2001;1:353–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
