Dysregulation of intracellular dopamine stores revealed in the R6/2 mouse striatum
- PMID: 19929911
- PMCID: PMC3999965
- DOI: 10.1111/j.1471-4159.2009.06501.x
Dysregulation of intracellular dopamine stores revealed in the R6/2 mouse striatum
Abstract
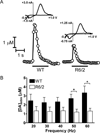
Huntington's disease (HD) is a fatal, neurodegenerative movement disorder characterized by preferential and extensive striatal degeneration. Here, we used fast-scan cyclic voltammetry to study the mobilization and efflux of reserve pool dopamine (DA) in striatal brain slices from HD model R6/2 mice. When applying stimulus trains of 120 pulses, evoked DA release in wild-type (WT) slices was greater than that in R6/2 slices at the higher frequencies (50 and 60 Hz). To quantify cytosolic and reserve pool DA levels, amphetamine-induced DA efflux was measured after pre-treatment with either tetrabenazine or alpha-methyl-p-tyrosine. Slices from 12-week-old R6/2 mice released less DA than slices from WT mice, while no difference was noted in slices from 6-week old mice. The vesicular release of reserve pool DA, mobilized by treatment with cocaine, was shorter lived in R6/2 slices compared with WT slices even though peak DA release was the same. Moreover, the number of DA reserve pool vesicles in R6/2 mice was less than half of that in WT. Therefore, our data suggest that the same number of DA molecules are present in each reserve pool vesicle in WT and R6/2 mice and that these vesicles are readily mobilized in both genotypes; however, R6/2 mice have fewer DA reserve pool vesicles available for mobilization.
Figures



Similar articles
-
Dopamine release is severely compromised in the R6/2 mouse model of Huntington's disease.J Neurochem. 2006 May;97(3):737-46. doi: 10.1111/j.1471-4159.2006.03762.x. Epub 2006 Mar 29. J Neurochem. 2006. PMID: 16573654
-
Impaired dopamine release and uptake in R6/1 Huntington's disease model mice.Neurosci Lett. 2011 Mar 29;492(1):11-4. doi: 10.1016/j.neulet.2011.01.036. Epub 2011 Jan 20. Neurosci Lett. 2011. PMID: 21256185 Free PMC article.
-
Dopamine-dependent long term potentiation in the dorsal striatum is reduced in the R6/2 mouse model of Huntington's disease.Neuroscience. 2007 Jun 8;146(4):1571-80. doi: 10.1016/j.neuroscience.2007.03.036. Epub 2007 May 2. Neuroscience. 2007. PMID: 17478055
-
Reduced striatal acetylcholine efflux in the R6/2 mouse model of Huntington's disease: an examination of the role of altered inhibitory and excitatory mechanisms.Exp Neurol. 2011 Dec;232(2):119-25. doi: 10.1016/j.expneurol.2011.08.010. Epub 2011 Aug 16. Exp Neurol. 2011. PMID: 21864528
-
Catecholamine exocytosis is diminished in R6/2 Huntington's disease model mice.J Neurochem. 2007 Dec;103(5):2102-10. doi: 10.1111/j.1471-4159.2007.04908.x. Epub 2007 Sep 14. J Neurochem. 2007. PMID: 17868298
Cited by
-
The Addenbrooke's Cognitive Examination-Revised accurately detects cognitive decline in Huntington's disease.J Neurol. 2013 Nov;260(11):2777-85. doi: 10.1007/s00415-013-7061-5. Epub 2013 Aug 7. J Neurol. 2013. PMID: 23922130
-
LC-MS/MS quantification of salvinorin A from biological fluids.Anal Methods. 2013 Dec 21;5(24):10.1039/C3AY40810H. doi: 10.1039/C3AY40810H. Anal Methods. 2013. PMID: 24416081 Free PMC article.
-
In vivo Dopamine Efflux is Decreased in Striatum of both Fragment (R6/2) and Full-Length (YAC128) Transgenic Mouse Models of Huntington's Disease.Front Syst Neurosci. 2011 Jul 15;5:61. doi: 10.3389/fnsys.2011.00061. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21811446 Free PMC article.
-
In Situ Electrochemical Monitoring of Caged Compound Photochemistry: An Internal Actinometer for Substrate Release.Anal Chem. 2021 Feb 9;93(5):2776-2784. doi: 10.1021/acs.analchem.0c03452. Epub 2021 Jan 25. Anal Chem. 2021. PMID: 33492927 Free PMC article.
-
Differential electrophysiological changes in striatal output neurons in Huntington's disease.J Neurosci. 2011 Jan 26;31(4):1170-82. doi: 10.1523/JNEUROSCI.3539-10.2011. J Neurosci. 2011. PMID: 21273402 Free PMC article.
References
-
- Abercrombie ED, Russo ML. Program Number 195.12. in Society for Neuroscience. 2002. Orlando, FL: 2002. Neurochemistry in the R6/2 Transgenic Mouse Model of Huntington's Disease.
-
- Bates GP, Harper PS, Jones L. Huntington's Disease. Oxford: Oxford University Press; 2002.
-
- Cooper JK, Schilling G, Peters MF, et al. Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture. Hum Mol Genet. 1998;7:783–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

