Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats
- PMID: 19929946
- PMCID: PMC4516553
- DOI: 10.1111/j.1582-4934.2009.00975.x
Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats
Abstract
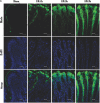
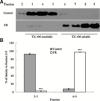
Tight junction (TJ) disruptions have been demonstrated both in vitro and more recently in vivo in infection. However, the molecular basis for changes of TJ during ischaemia-reperfusion (I/R) injury is poorly understood. In the present study, intestinal damage was induced by I/R in an animal model. As assessed by TUNEL and propidium iodide uptake, we showed that I/R injury induced apoptosis as well as necrosis in rat colon, and the frequency of apoptotic and necrotic cells reached the maximum at 5 hrs of reperfusion. Immunofluorescence microscopy revealed that claudins 1, 3 and 5 are strongly expressed in the surface epithelial cells of the colon; however, labelling of all three proteins was present diffusely within cells and no longer focused at the lateral cell boundaries after I/R. Using Western blot analysis, we found that distribution of TJ proteins in membrane microdomains of TJ was markedly affected in I/R injury rats. Occludin, ZO-1, claudin-1 and claudin-3 were completely displaced from TX-100 insoluble fractions to TX-100 soluble fractions, and claudin-5 was partly displaced. The distribution of lipid raft marker protein caveolin-1 was also changed after I/R. I/R injury results in the disruption of TJs, which characterized by relocalization of the claudins 1, 3 and 5 and an increase in intestinal permeability using molecular tracer measurement. I/R injury altered distribution of TJ proteins in vivo that was associated with functional TJ deficiencies.
Figures
















References
-
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. - PubMed
-
- Hawker PC, McKay JS, Turnberg LA. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980;79:508–11. - PubMed
-
- Sandle GI, Higgs N, Crowe P. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. - PubMed
-
- Gassler N, Rohr C, Schneider A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

