Role for LAMP-2 in endosomal cholesterol transport
- PMID: 19929948
- PMCID: PMC3822795
- DOI: 10.1111/j.1582-4934.2009.00973.x
Role for LAMP-2 in endosomal cholesterol transport
Abstract
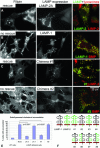
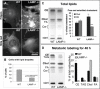
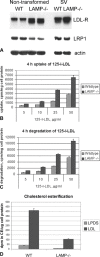
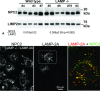
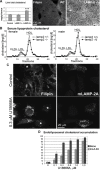
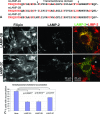
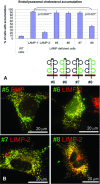
The mechanisms of endosomal and lysosomal cholesterol traffic are still poorly understood. We showed previously that unesterified cholesterol accumulates in the late endosomes and lysosomes of fibroblasts deficient in both lysosome associated membrane protein-2 (LAMP-2) and LAMP-1, two abundant membrane proteins of late endosomes and lysosomes. In this study we show that in cells deficient in both LAMP-1 and LAMP-2 (LAMP(-/-)), low-density lipoprotein (LDL) receptor levels and LDL uptake are increased as compared to wild-type cells. However, there is a defect in esterification of both endogenous and LDL cholesterol. These results suggest that LAMP(-/-) cells have a defect in cholesterol transport to the site of esterification in the endoplasmic reticulum, likely due to defective export of cholesterol out of late endosomes or lysosomes. We also show that cholesterol accumulates in LAMP-2 deficient liver and that overexpression of LAMP-2 retards the lysosomal cholesterol accumulation induced by U18666A. These results point to a critical role for LAMP-2 in endosomal/lysosomal cholesterol export. Moreover, the late endosomal/lysosomal cholesterol accumulation in LAMP(-/-) cells was diminished by overexpression of any of the three isoforms of LAMP-2, but not by LAMP-1. The LAMP-2 luminal domain, the membrane-proximal half in particular, was necessary and sufficient for the rescue effect. Taken together, our results suggest that LAMP-2, its luminal domain in particular, plays a critical role in endosomal cholesterol transport and that this is distinct from the chaperone-mediated autophagy function of LAMP-2.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
References
-
- Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lyso-somal membrane proteins. Trends Cell Biol. 2003;13:137–45. - PubMed
-
- Schröder B, Wrocklage C, Pan C, et al. Integral and associated lysosomal membrane proteins. Traffic. 2007;8:1676–86. - PubMed
-
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–89. - PubMed
-
- Fukuda M. Lysosomal membrane glyco-proteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991;266:21327–30. - PubMed
-
- Carlsson SR, Roth J, Piller F, et al. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglyco-proteins carrying polylactosaminoglycan. J Biol Chem. 1988;263:18911–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

