Integrated-boost IMRT or 3-D-CRT using FET-PET based auto-contoured target volume delineation for glioblastoma multiforme--a dosimetric comparison
- PMID: 19930657
- PMCID: PMC2787527
- DOI: 10.1186/1748-717X-4-57
Integrated-boost IMRT or 3-D-CRT using FET-PET based auto-contoured target volume delineation for glioblastoma multiforme--a dosimetric comparison
Abstract
Background: Biological brain tumor imaging using O-(2-[18F]fluoroethyl)-L-tyrosine (FET)-PET combined with inverse treatment planning for locally restricted dose escalation in patients with glioblastoma multiforme seems to be a promising approach.The aim of this study was to compare inverse with forward treatment planning for an integrated boost dose application in patients suffering from a glioblastoma multiforme, while biological target volumes are based on FET-PET and MRI data sets.
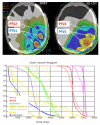
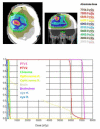
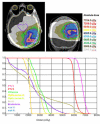
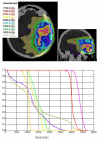
Methods: In 16 glioblastoma patients an intensity-modulated radiotherapy technique comprising an integrated boost (IB-IMRT) and a 3-dimensional conventional radiotherapy (3D-CRT) technique were generated for dosimetric comparison. FET-PET, MRI and treatment planning CT (P-CT) were co-registrated. The integrated boost volume (PTV1) was auto-contoured using a cut-off tumor-to-brain ratio (TBR) of > or = 1.6 from FET-PET. PTV2 delineation was MRI-based. The total dose was prescribed to 72 and 60 Gy for PTV1 and PTV2, using daily fractions of 2.4 and 2 Gy.
Results: After auto-contouring of PTV1 a marked target shape complexity had an impact on the dosimetric outcome. Patients with 3-4 PTV1 subvolumes vs. a single volume revealed a significant decrease in mean dose (67.7 vs. 70.6 Gy). From convex to complex shaped PTV1 mean doses decreased from 71.3 Gy to 67.7 Gy. The homogeneity and conformity for PTV1 and PTV2 was significantly improved with IB-IMRT. With the use of IB-IMRT the minimum dose within PTV1 (61.1 vs. 57.4 Gy) and PTV2 (51.4 vs. 40.9 Gy) increased significantly, and the mean EUD for PTV2 was improved (59.9 vs. 55.3 Gy, p < 0.01). The EUD for PTV1 was only slightly improved (68.3 vs. 67.3 Gy). The EUD for the brain was equal with both planning techniques.
Conclusion: In the presented planning study the integrated boost concept based on inversely planned IB-IMRT is feasible. The FET-PET-based automatically contoured PTV1 can lead to very complex geometric configurations, limiting the achievable mean dose in the boost volume. With IB-IMRT a better homogeneity and conformity, compared to 3D-CRT, could be achieved.
Figures
References
-
- Stupp R, Hegi ME, Mason WP, Bent MJ van den, Taphoorn MJ, Janzer RC. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–1731. - PubMed
-
- Taghian A, Ramsay J, Allalunis-Turner J, Budach W, Gioioso D, Pardo F. Intrinsic radiation sensitivity may not be the major determinant of the poor clinical outcome of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1993;25(2):243–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

