PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling
- PMID: 19930715
- PMCID: PMC2792223
- DOI: 10.1186/1476-4598-8-110
PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling
Abstract
Background: Phosphatase of regenerating liver-3 (PRL-3) plays a causative role in tumor metastasis, but the underlying mechanisms are not well understood. In our previous study, we observed that PRL-3 could decrease tyrosine phosphorylation of integrin beta1 and enhance activation of ERK1/2 in HEK293 cells. Herein we aim to explore the association of PRL-3 with integrin beta1 signaling and its functional implications in motility, invasion, and metastasis of colon cancer cell LoVo.
Methods: Transwell chamber assay and nude mouse model were used to study motility and invasion, and metastsis of LoVo colon cancer cells, respectively. Knockdown of integrin beta1 by siRNA or lentivirus were detected with Western blot and RT-PCR. The effect of PRL-3 on integrin beta1, ERK1/2, and MMPs that mediate motility, invasion, and metastasis were measured by Western blot, immunofluorencence, co-immunoprecipitation and zymographic assays.
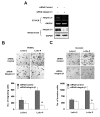
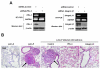
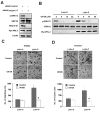
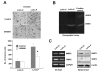
Results: We demonstrated that PRL-3 associated with integrin beta1 and its expression was positively correlated with ERK1/2 phosphorylation in colon cancer tissues. Depletion of integrin beta1 with siRNA, not only abrogated the activation of ERK1/2 stimulated by PRL-3, but also abolished PRL-3-induced motility and invasion of LoVo cells in vitro. Similarly, inhibition of ERK1/2 phosphorylation with U0126 or MMP activity with GM6001 also impaired PRL-3-induced invasion. In addition, PRL-3 promoted gelatinolytic activity of MMP2, and this stimulation correlated with decreased TIMP2 expression. Moreover, PRL-3-stimulated lung metastasis of LoVo cells in a nude mouse model was inhibited when integrin beta1 expression was interfered with shRNA.
Conclusion: Our results suggest that PRL-3's roles in motility, invasion, and metastasis in colon cancer are critically controlled by the integrin beta1-ERK1/2-MMP2 signaling.
Figures


Similar articles
-
Proteomic analysis identifies translationally controlled tumor protein as a mediator of phosphatase of regenerating liver-3-promoted proliferation, migration and invasion in human colon cancer cells.Chin Med J (Engl). 2011 Nov;124(22):3778-85. Chin Med J (Engl). 2011. PMID: 22340241
-
Phosphatase of regenerating liver-3 promotes migration and invasion by upregulating matrix metalloproteinases-7 in human colorectal cancer cells.Int J Cancer. 2012 Aug 1;131(3):E190-203. doi: 10.1002/ijc.27381. Epub 2012 Jan 11. Int J Cancer. 2012. PMID: 22131018
-
Extracellular gamma-synuclein promotes tumor cell motility by activating β1 integrin-focal adhesion kinase signaling pathway and increasing matrix metalloproteinase-24, -2 protein secretion.J Exp Clin Cancer Res. 2018 Jun 15;37(1):117. doi: 10.1186/s13046-018-0783-6. J Exp Clin Cancer Res. 2018. PMID: 29903032 Free PMC article.
-
PRL phosphatases as potential molecular targets in cancer.Mol Cancer Ther. 2005 Nov;4(11):1653-61. doi: 10.1158/1535-7163.MCT-05-0248. Mol Cancer Ther. 2005. PMID: 16275986 Review.
-
Phosphatase of regenerating liver: a novel target for cancer therapy.Expert Opin Ther Targets. 2014 May;18(5):555-69. doi: 10.1517/14728222.2014.892926. Epub 2014 Mar 1. Expert Opin Ther Targets. 2014. PMID: 24579927 Free PMC article. Review.
Cited by
-
PRL-3 promotes migration and invasion and is associated with poor prognosis in salivary adenoid cystic carcinoma.J Oral Pathol Med. 2016 Feb;45(2):111-8. doi: 10.1111/jop.12331. Epub 2015 Jun 3. J Oral Pathol Med. 2016. PMID: 26041460 Free PMC article.
-
PRL-3 promotes the peritoneal metastasis of gastric cancer through the PI3K/Akt signaling pathway by regulating PTEN.Oncol Rep. 2016 Oct;36(4):1819-28. doi: 10.3892/or.2016.5030. Epub 2016 Aug 23. Oncol Rep. 2016. PMID: 27572739 Free PMC article.
-
Phosphatase of regenerating liver 3 (PRL3) provokes a tyrosine phosphoproteome to drive prometastatic signal transduction.Mol Cell Proteomics. 2013 Dec;12(12):3759-77. doi: 10.1074/mcp.M113.028886. Epub 2013 Sep 12. Mol Cell Proteomics. 2013. PMID: 24030100 Free PMC article.
-
Development and characterization of nanobodies that specifically target the oncogenic Phosphatase of Regenerating Liver-3 (PRL-3) and impact its interaction with a known binding partner, CNNM3.PLoS One. 2023 May 23;18(5):e0285964. doi: 10.1371/journal.pone.0285964. eCollection 2023. PLoS One. 2023. PMID: 37220097 Free PMC article.
-
Cucurbitacin B inhibits cell proliferation and induces apoptosis in human osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK pathways.Exp Ther Med. 2017 Jul;14(1):805-812. doi: 10.3892/etm.2017.4547. Epub 2017 Jun 6. Exp Ther Med. 2017. PMID: 28673003 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

