The interaction of angiocidin with tissue transglutaminase
- PMID: 19931242
- PMCID: PMC2815215
- DOI: 10.1016/j.yexmp.2009.11.001
The interaction of angiocidin with tissue transglutaminase
Abstract
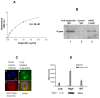
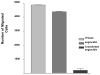
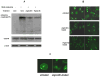
Angiocidin, a matrix bound and tumor associated protein, has been shown to inhibit tumor progression and angiogenesis. We previously demonstrated that angiocidin binds to thrombospondin-1 and alpha2beta1 integrin. We now show that angiocidin binds and is a preferred substrate for tissue transglutaminase-2 (tTgase). Angiocidin bound tTgase saturably with a Kd of 26 nM, while an angiocidin deletion mutant missing the matrix binding domain of angiocidin failed to bind tTgase. tTgase colocalized with angiocidin on endothelial cells. tTgase bound anti-angiocidin immunoprecipitates of endothelial cell lysates. Breast cancer cells expressing high levels of tTgase attached to angiocidin immobilized on tissue culture plates. Angiocidin was a preferred substrate for tTgase forming high molecular weight cross-linked multimers when treated with tTgase. Cross-linked angiocidin contained iso-peptide bonds as demonstrated by Western blotting and immunohistochemical colocalization studies using endothelial cells treated with angiocidin. Cross-linked angiocidin inhibited cell migration in contrast to monomeric angiocidin and inhibited localization of fibronectin (FN), a pro-tumorigenic matrix protein, into the extracellular matrix (ECM) of tumor and HUVE cells. Our studies provide an additional explanation for the anti-tumor activity of angiocidin suggesting that cross-linked angiocidin disrupts the tumor ECM making it less permissive for tumor growth.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Integrin alpha2beta1 mediates the anti-angiogenic and anti-tumor activities of angiocidin, a novel tumor-associated protein.Exp Cell Res. 2006 Aug 1;312(13):2443-53. doi: 10.1016/j.yexcr.2006.04.009. Epub 2006 Apr 30. Exp Cell Res. 2006. PMID: 16762342
-
Analysis of tissue transglutaminase function in the migration of Swiss 3T3 fibroblasts: the active-state conformation of the enzyme does not affect cell motility but is important for its secretion.J Biol Chem. 2002 May 10;277(19):16567-75. doi: 10.1074/jbc.M109836200. Epub 2002 Feb 26. J Biol Chem. 2002. PMID: 11867617
-
Regulation of cell surface tissue transglutaminase: effects on matrix storage of latent transforming growth factor-beta binding protein-1.J Histochem Cytochem. 1999 Nov;47(11):1417-32. doi: 10.1177/002215549904701108. J Histochem Cytochem. 1999. PMID: 10544215
-
Tissue transglutaminase in tumour progression: friend or foe?Amino Acids. 2007 Aug;33(2):373-84. doi: 10.1007/s00726-007-0516-1. Epub 2007 Jun 20. Amino Acids. 2007. PMID: 17581697 Review.
-
Tissue transglutaminase in normal and abnormal wound healing: review article.Amino Acids. 2004 Jul;26(4):387-404. doi: 10.1007/s00726-004-0094-4. Epub 2004 Jun 21. Amino Acids. 2004. PMID: 15290345 Review.
Cited by
-
Reduction of angiocidin contributes to decreased HepG2 cell proliferation.Afr Health Sci. 2013 Sep;13(3):560-4. doi: 10.4314/ahs.v13i3.5. Afr Health Sci. 2013. PMID: 24250289 Free PMC article.
-
Angiocidin inhibits breast cancer proliferation through activation of epidermal growth factor receptor and nuclear factor kappa (NF-ĸB).Exp Mol Pathol. 2011 Jun;90(3):244-51. doi: 10.1016/j.yexmp.2011.01.002. Epub 2011 Jan 15. Exp Mol Pathol. 2011. PMID: 21241690 Free PMC article.
-
Cellular functions of tissue transglutaminase.Int Rev Cell Mol Biol. 2012;294:1-97. doi: 10.1016/B978-0-12-394305-7.00001-X. Int Rev Cell Mol Biol. 2012. PMID: 22364871 Free PMC article. Review.
-
Physiological, pathological, and structural implications of non-enzymatic protein-protein interactions of the multifunctional human transglutaminase 2.Cell Mol Life Sci. 2015 Aug;72(16):3009-35. doi: 10.1007/s00018-015-1909-z. Epub 2015 May 6. Cell Mol Life Sci. 2015. PMID: 25943306 Free PMC article. Review.
-
Extracellular TG2: emerging functions and regulation.FEBS J. 2011 Dec;278(24):4704-16. doi: 10.1111/j.1742-4658.2011.08346.x. Epub 2011 Nov 21. FEBS J. 2011. PMID: 21902810 Free PMC article. Review.
References
-
- Aeschlimann D, Paulsson M. Cross-linking of laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilization. J Biol Chem. 1991;266:15308–17. - PubMed
-
- Arnoletti JP, et al. Computer-assisted image analysis of tumor sections for a new thrombospondin receptor. Am J Surg. 1994;168:433–6. - PubMed
-
- Bale MD, Mosher DF. Thrombospondin is a substrate for blood coagulation factor XIIIa. Biochemistry. 1986;25:5667–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

