Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice
- PMID: 19931266
- PMCID: PMC2831148
- DOI: 10.1053/j.gastro.2009.11.043
Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice
Abstract
Background & aims: Helicobacter pylori infection increases gastric regulatory T cell (Treg) response, which may contribute to H pylori immune escape. We hypothesize that H pylori directs Treg skewing by way of dendritic cells (DCs) and thus inhibits interleukin-17(+) helper T cells (Th17) immunity.
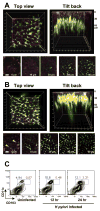
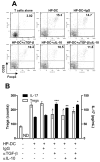
Methods: Two-photon microscopy was used to locate DCs in gastric lamina propria of mice. The induction of Th17 and Treg responses by bacteria-pulsed murine bone marrow-derived DCs was analyzed by cytokine production and stimulation of T-cell proliferation. The effect of VacA, CagA, transforming growth factor-beta (TGF-beta), and IL-10 on Th17/Treg balance was assessed. The in vivo significance of Tregs on the H pylori-specific Th17 response and H pylori density was determined by using anti-CD25 neutralizing antibodies to deplete Tregs in mice.
Results: We showed that mucosal CD11c(+) DCs are located near the surface of normal gastric epithelium, and their number increased after H pylori infection. Study of the direct interaction of DCs with H pylori showed a Treg-skewed response. The Treg skewing was independent of H pylori VacA and CagA and dependent on TGF-beta and IL-10. In vivo Treg skewing by adoptive transfer of H pylori-pulsed DCs reduces the ratio of gastric IL-17/Foxp3 mRNA expressions. The depletion of CD25(+) Tregs results in early reduction of H pylori density, which is correlated with enhanced peripheral H pylori-specific Th17, but not Th1, response.
Conclusions: Overall, our study indicates that H pylori alters the DC-polarized Th17/Treg balance toward a Treg-biased response, which suppresses the effective induction of H pylori-specific Th17 immunity.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Perez-Perez GI, Dworkin BM, Chodos JE, et al. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11–7. - PubMed
-
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. - PubMed
-
- Cooke CL, Huff JL, Solnick JV. The role of genome diversity and immune evasion in persistent infection with Helicobacter pylori. FEMS Immunol Med Microbiol. 2005;45:11–23. - PubMed
-
- Raghavan S, Suri-Payer E, Holmgren J. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4CD25 regulatory T cells. Scand J Immunol. 2004;60:82–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

